
Study Guide with Student Solutions Manual for McMurry's Organic Chemistry, 9th
9th Edition
ISBN: 9781305082144
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2.SE, Problem 21VC
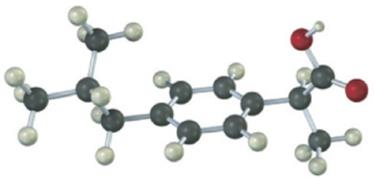
The following model is a representation of ibuprofen, a common over- the-counter pain reliever. Indicate the positions of the multiple bonds, and draw a skeletal structure (gray = C, red = O, ivory = H).
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Learning Goal:
This question reviews the format for writing an element's written symbol. Recall that written symbols have a particular format. Written symbols use a form like this:
35 Cl
17
In this form the mass number, 35, is a stacked superscript. The atomic number, 17, is a stacked subscript. "CI" is the chemical symbol for the element chlorine. A general way to show this form is:
It is also correct to write symbols by leaving off the atomic number, as in the following form:
atomic number
mass number Symbol
35 Cl or
mass number Symbol
This is because if you write the element symbol, such as Cl, you know the atomic number is 17 from that symbol. Remember that the atomic number, or number of protons in the nucleus, is what defines the element. Thus, if 17 protons
are in the nucleus, the element can only be chlorine. Sometimes you will only see 35 C1, where the atomic number is not written.
Watch this video to review the format for written symbols.
In the following table each column…
need help please and thanks dont understand only need help with C-F
Learning Goal:
As discussed during the lecture, the enzyme HIV-1 reverse transcriptae (HIV-RT) plays a significant role for the HIV virus and is an important drug target. Assume a concentration [E] of 2.00 µM (i.e. 2.00 x 10-6 mol/l) for HIV-RT. Two potential drug molecules, D1 and D2, were identified, which form stable complexes with the HIV-RT.
The dissociation constant of the complex ED1 formed by HIV-RT and the drug D1 is 1.00 nM (i.e. 1.00 x 10-9). The dissociation constant of the complex ED2 formed by HIV-RT and the drug D2 is 100 nM (i.e. 1.00 x 10-7).
Part A - Difference in binding free eenergies
Compute the difference in binding free energy (at a physiological temperature T=310 K) for the complexes. Provide the difference as a positive numerical expression with three significant figures in kJ/mol.
The margin of error is 2%.
Part B - Compare difference in free energy to the thermal…
need help please and thanks dont understand only need help with C-F
Learning Goal:
As discussed during the lecture, the enzyme HIV-1 reverse transcriptae (HIV-RT) plays a significant role for the HIV virus and is an important drug target. Assume a concentration [E] of 2.00 µM (i.e. 2.00 x 10-6 mol/l) for HIV-RT. Two potential drug molecules, D1 and D2, were identified, which form stable complexes with the HIV-RT.
The dissociation constant of the complex ED1 formed by HIV-RT and the drug D1 is 1.00 nM (i.e. 1.00 x 10-9). The dissociation constant of the complex ED2 formed by HIV-RT and the drug D2 is 100 nM (i.e. 1.00 x 10-7).
Part A - Difference in binding free eenergies
Compute the difference in binding free energy (at a physiological temperature T=310 K) for the complexes. Provide the difference as a positive numerical expression with three significant figures in kJ/mol.
The margin of error is 2%.
Part B - Compare difference in free energy to the thermal…
Chapter 2 Solutions
Study Guide with Student Solutions Manual for McMurry's Organic Chemistry, 9th
Ch. 2.1 - Prob. 1PCh. 2.1 - Prob. 2PCh. 2.1 - Use the electronegativity values shown in Figure...Ch. 2.1 - Look at the following electrostatic potential map...Ch. 2.2 - Ethylene glycol, HOCH2CH2OH, may look nonpolar...Ch. 2.2 - Make three-dimensional drawings of the following...Ch. 2.3 - Calculate formal charges for the nonhydrogen atoms...Ch. 2.3 - Organic phosphate groups occur commonly in...Ch. 2.6 - Which of the following pairs of structures...Ch. 2.6 - Draw the indicated number of resonance forms for...
Ch. 2.7 - Nitric acid (HNO3) reacts with ammonia (NH3) to...Ch. 2.8 - Prob. 12PCh. 2.8 - Amide ion, H2N-, is a much stronger base than...Ch. 2.9 - Prob. 14PCh. 2.9 - Prob. 15PCh. 2.9 - Prob. 16PCh. 2.11 - Using curved arrows, show how the species in part...Ch. 2.11 - Prob. 18PCh. 2.12 - Of the two vitamins A and C, one is hydrophilic...Ch. 2.SE - Prob. 20VCCh. 2.SE - The following model is a representation of...Ch. 2.SE - cis-l, 2-Dichloroethylene and trans-1,...Ch. 2.SE - The following molecular models are representations...Ch. 2.SE - Predict the product(s) of the acid/base reactions...Ch. 2.SE - Use curved arrows to draw the protonated form of...Ch. 2.SE - Prob. 26MPCh. 2.SE - Double bonds can also act like Lewis bases,...Ch. 2.SE - Prob. 28APCh. 2.SE - Use the electronegativity table given in Figure...Ch. 2.SE - Which of the following molecules has a dipole...Ch. 2.SE - Prob. 31APCh. 2.SE - Phosgene, C12C=O, has a smaller dipole moment than...Ch. 2.SE - Prob. 33APCh. 2.SE - Methanethiol, CH3SH, has a substantial dipole...Ch. 2.SE - Calculate the formal charges on the atoms shown in...Ch. 2.SE - Assign formal charges to the atoms in each of the...Ch. 2.SE - Which of the following pairs of structures...Ch. 2.SE - Prob. 38APCh. 2.SE - 1, 3-Cyclobutadiene is a rectangular molecule with...Ch. 2.SE - Alcohols can act either as weak acids or as weak...Ch. 2.SE - The O-H hydrogen in acetic acid is more acidic...Ch. 2.SE - Draw electron-dot structures for the following...Ch. 2.SE - Write the products of the following acid-base...Ch. 2.SE - Rank the following substances in order of...Ch. 2.SE - Which, if any, of the substances in Problem 2-44...Ch. 2.SE - The ammonium ion (NH4+, pKa = 9.25) has a lower...Ch. 2.SE - Prob. 47APCh. 2.SE - Prob. 48APCh. 2.SE - Calculate Ka values from the following pka’s:...Ch. 2.SE - Calculate pKa values from the following Ka’s:...Ch. 2.SE - What is the pH of a 0.050 M solution of formic...Ch. 2.SE - Prob. 52APCh. 2.SE - Maleic acid has a dipole moment, but the closely...Ch. 2.SE - Assume that you have two unlabeled bottles, one of...Ch. 2.SE - Identify the acids and bases in the following...Ch. 2.SE - Which of the following pairs represent resonance...Ch. 2.SE - Draw as many resonance structures as you can for...Ch. 2.SE - Carbocations, which contain a trivalent,...Ch. 2.SE - We’ll see in the next chapter that organic...Ch. 2.SE - The azide functional group, which occurs in...Ch. 2.SE - Phenol, C6H5OH, is a stronger acid than methanol,...Ch. 2.SE - Thiamin diphosphate (TPP), a derivative of vitamin...Ch. 2.SE - Determine if each compound or ion below has a...Ch. 2.SE - Prob. 64APCh. 2.SE - Prob. 65APCh. 2.SE - Draw the conjugate base for each compound below...Ch. 2.SE - 1, 1, 1-Trichloroethanol is an acid more than 1000...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please correct answer and don't used hand raitingarrow_forwardneed help please and thanks dont understand a-b Learning Goal: As discussed during the lecture, the enzyme HIV-1 reverse transcriptae (HIV-RT) plays a significant role for the HIV virus and is an important drug target. Assume a concentration [E] of 2.00 µM (i.e. 2.00 x 10-6 mol/l) for HIV-RT. Two potential drug molecules, D1 and D2, were identified, which form stable complexes with the HIV-RT. The dissociation constant of the complex ED1 formed by HIV-RT and the drug D1 is 1.00 nM (i.e. 1.00 x 10-9). The dissociation constant of the complex ED2 formed by HIV-RT and the drug D2 is 100 nM (i.e. 1.00 x 10-7). Part A - Difference in binding free eenergies Compute the difference in binding free energy (at a physiological temperature T=310 K) for the complexes. Provide the difference as a positive numerical expression with three significant figures in kJ/mol. The margin of error is 2%. Part B - Compare difference in free energy to the thermal energy Divide the…arrow_forwardPlease correct answer and don't used hand raitingarrow_forward
- Please correct answer and don't used hand raitingarrow_forwardCan you tell me if my answers are correctarrow_forwardBunsenite (NiO) crystallizes like common salt (NaCl), with a lattice parameter a = 4.177 Å. A sample of this mineral that has Schottky defects that are not supposed to decrease the volume of the material has a density of 6.67 g/cm3. What percentage of NiO molecules is missing? (Data: atomic weight of Ni: 58.7; atomic weight of O: 16).arrow_forward
- A sample of aluminum (face-centered cubic - FCC) has a density of 2.695 mg/m3 and a lattice parameter of 4.04958 Å. Calculate the fraction of vacancies in the structure. (Atomic weight of aluminum: 26.981).arrow_forwardPlease correct answer and don't used hand raitingarrow_forwardPlease correct answer and don't used hand raitingarrow_forward
- Please correct answer and don't used hand raitingarrow_forwardWhich of the following species is a valid resonance structure of A? Use curved arrows to show how A is converted to any valid resonance structure. When a compound is not a valid resonance structurc of A, explain why not. Provide steps and tips on what to look for to understand how to solve and apply to other problems.arrow_forwardN IZ Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 HN Molecule 3 Х HN www. Molecule 4 Molecule 5 Molecule 6 none of the above NH NH Garrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

Types of bonds; Author: Edspira;https://www.youtube.com/watch?v=Jj0V01Arebk;License: Standard YouTube License, CC-BY