
(a)
To determine:
Whether a H bond will be formed in a reaction between H3C-CH=O and H2O with the help of a figure.
Introduction:
(b)
To determine:
With the help of diagrams if the molecules Cl2, NH3 and CH4 will be polar or not.
Introduction:
When atoms combine to form molecules, they form chemical bonds by sharing, donating or accepting electrons. In case of sharing, the combining atoms may or may not share the electrons equally. If one of the atoms is more electronegative, it pulls the shared electron cloud towards itself and acquires negative charge. The other atom thus acquires positive charge. Such a molecule is called polar.
(c)
To determine:
The pH of a solution with a H+ concentration of 0.00001 M.
Introduction:
Dissociation of water results in generation of H+ and OH- ions. However, these ions are equal in number. Acids are compounds that release H+ in solution and bases are compounds that release OH- in solution. The pH scale expresses the concentration of H+ ions in a solution. pH less than 7 is indicative of acidic solution and pH above 7 indicates a basic (alkaline) solution. pH 7 is an indicator of a neutral solution.
(d)
To determine:
The pH of a solution with a OH- concentration of 0.00001 M.
Introduction:
Dissociation of water results in generation of H+ and OH- ions. However, these ions are equal in number. Acids are compounds that release H+ in solution and bases are compounds that release OH- in solution. The pH scale expresses the concentration of H+ ions in a solution. pH less than 7 is indicative of acidic solution and pH above 7 indicates a basic (alkaline) solution. pH 7 is an indicator of a neutral solution.
(e)
To determine:
The kinds of bonds that magnesium can make based on the diagram of its atomic structure.
Introduction:
Atoms combine with other atoms by making chemical bonds with them. These bonds can be made by either sharing, donating or accepting electrons. The number of electrons in the outer-most orbital of an atom determine its tendency to form bonds. These electrons are called valence electrons. Chemical bonds are formed in a way that the outer-most orbital is completely filled with electrons.
(f)
To determine:
The kind of ion that Magnesium would make, depending on its valence.
Introduction:
Atoms combine with other atoms by making chemical bonds with them. These bonds can be made by either sharing, donating or accepting electrons. The number of electrons in the outer-most orbital of an atom determine its tendency to form bonds. These electrons are called valence electrons. Chemical bonds are formed in a way that the outer-most orbital is completely filled with electrons.
Explanation:
Looking at the atomic structure of Magnesium, it is observed that the 12 electrons are distributed is different orbital as 2, 8 and 2 electrons. The outer-most orbital has 2 electrons. Thus, Mg forms bonds by giving up its two valence electrons to atoms that may accept them. Thus, Mg loses 2 electrons to become positively charged ion Mg2+. Positively charged ions are also known as cations.
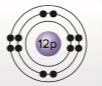
Conclusion:
Magnesium loses two electrons from its outer-most orbital to gain positive charge on its ion.
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
Foundations In Microbiology: Basic Principles (10th International Edition)
- When setting up a PCR reaction to act as a negative control for the surface protein A gene... Which primers will you add to the reaction mix? mecA primers, spa primers, mecA primers and spa primers, no primers What will you add in place of template? sterile water, MRSA DNA, Patient DNA, S. aureus DNAarrow_forwardDraft a science fair project for a 11 year old based on the human body, specifically the liverarrow_forwardYou generate a transgenic mouse line with a lox-stop-lox sequence upstream of a dominant-negative Notch fused to GFP. Upon crossing this mouse with another mouse line expressing ectoderm-specific Cre, what would you expect for the phenotype of neuronal differentiation in the resulting embryos?arrow_forward
- Hair follicle formation is thought to result from a reaction-diffusion mechanism with Wnt and its antagonist Dkk1. How is Dkk1 regulated by Wnt? Describe specific cis-regulatory elements and the net effect on Dkk1 expression.arrow_forwardLimetown S1E4 Transcript: E n 2025SP-BIO-111-PSNT1: Natu X Natural Selection in insects X + newconnect.mheducation.com/student/todo CA NATURAL SELECTION NATURAL SELECTION IN INSECTS (HARDY-WEINBERG LAW) INTRODUCTION LABORATORY SIMULATION A Lab Data Is this the correct allele frequency? Is this the correct genotype frequency? Is this the correct phenotype frequency? Total 1000 Phenotype Frequency Typica Carbonaria Allele Frequency 9 P 635 823 968 1118 1435 Color Initial Frequency Light 0.25 Dark 0.75 Frequency Gs 0.02 Allele Initial Allele Frequency Gs Allele Frequency d 0.50 0 D 0.50 0 Genotype Frequency Moths Genotype Color Moths Released Initial Frequency Frequency G5 Number of Moths Gs NC - Xarrow_forwardWhich of the following is not a sequence-specific DNA binding protein? 1. the catabolite-activated protein 2. the trp repressor protein 3. the flowering locus C protein 4. the flowering locus D protein 5. GAL4 6. all of the above are sequence-specific DNA binding proteinsarrow_forward
- Which of the following is not a DNA binding protein? 1. the lac repressor protein 2. the catabolite activated protein 3. the trp repressor protein 4. the flowering locus C protein 5. the flowering locus D protein 6. GAL4 7. all of the above are DNA binding proteinsarrow_forwardWhat symbolic and cultural behaviors are evident in the archaeological record and associated with Neandertals and anatomically modern humans in Europe beginning around 35,000 yBP (during the Upper Paleolithic)?arrow_forwardDescribe three cranial and postcranial features of Neanderthals skeletons that are likely adaptation to the cold climates of Upper Pleistocene Europe and explain how they are adaptations to a cold climate.arrow_forward
- Biology Questionarrow_forward✓ Details Draw a protein that is embedded in a membrane (a transmembrane protein), label the lipid bilayer and the protein. Identify the areas of the lipid bilayer that are hydrophobic and hydrophilic. Draw a membrane with two transporters: a proton pump transporter that uses ATP to generate a proton gradient, and a second transporter that moves glucose by secondary active transport (cartoon-like is ok). It will be important to show protons moving in the correct direction, and that the transporter that is powered by secondary active transport is logically related to the proton pump.arrow_forwarddrawing chemical structure of ATP. please draw in and label whats asked. Thank you.arrow_forward
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning





