
Concept explainers
Interpretation:
The amino acid tyrosine is biologically degraded by a series of steps that include the following transformations:
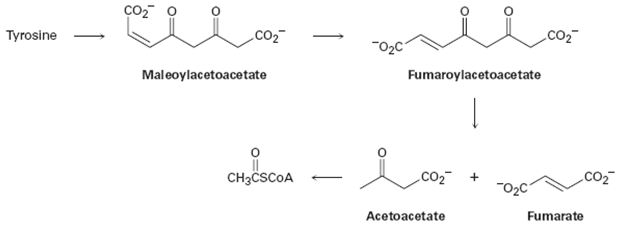
Concept introduction:
α-amino acid, in addition to their role as proton monomeric units, are energy
1) Essential amino acid
2) Non essential amino acid
While the non essential amino acid can be synchronized from metabolic processor, the essential amino acid must be provide by the diet. However, the essential dietary amino acid, instead of being extract or stored for future use, are converted to common metabolic intermediate like pyruvate, oxaloacetate, acetyl CoA, α-ketoglutarate etc. Thus amino acid are also precious of glucose, fatty acid, and
For the present, tyrosine clarifies itself to be a non essential amino acid, it is thus a metabolic processor and need not be supplied by the diet.
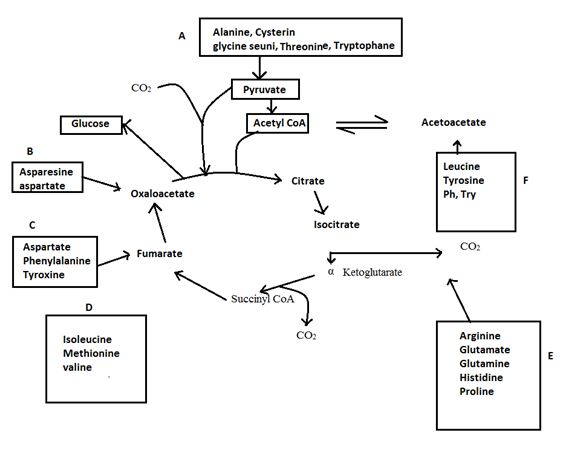
Further tyrisone is alos both a glucogenic and ketogenic amino acid. Its carbon skeleton can be degraded to either of pyruvate, α-ketoglutarate acetyl CoA, fumarate or oxaloacetate glucogenic amino acid and also to acetyl CoA or aceto acetate to be converted to ketone bodies or fatty acid. (ketogenic amino acid)
Degradation of amino acids to one of seven common metabolic intermediates
Want to see the full answer?
Check out a sample textbook solution
Chapter 29 Solutions
OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card for McMurry's Organic Chemistry, 9th
- Calculate the chemical shifts in 13C and 1H NMR for 4-chloropropiophenone ? Write structure and label hydrogens and carbonsarrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuu, don't solve it by AI plleeaasseeearrow_forward
- Please sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forward4. Read paragraph 4.15 from your textbook, use your calculated lattice energy values for CuO, CuCO3 and Cu(OH)2 an explain thermal decomposition reaction of malachite: Cu2CO3(OH)2 →2CuO + H2O + CO2 (3 points)arrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forward
- III O Organic Chemistry Using wedges and dashes in skeletal structures Draw a skeletal ("line") structure for each of the molecules below. Be sure your structures show the important difference between the molecules. key O O O O O CHON Cl jiii iiiiiiii You can drag the slider to rotate the molecules. Explanation Check Click and drag to start drawing a structure. Q Search X G ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use F 3 W C 3/5arrow_forward3. Use Kapustinskii's equation and data from Table 4.10 in your textbook to calculate lattice energies of Cu(OH)2 and CuCO3 (4 points)arrow_forward2. Copper (II) oxide crystalizes in monoclinic unit cell (included below; blue spheres 2+ represent Cu²+, red - O²-). Use Kapustinski's equation (4.5) to calculate lattice energy for CuO. You will need some data from Resource section of your textbook (p.901). (4 points) CuOarrow_forward
- What is the IUPAC name of the following compound? OH (2S, 4R)-4-chloropentan-2-ol O (2R, 4R)-4-chloropentan-2-ol O (2R, 4S)-4-chloropentan-2-ol O(2S, 4S)-4-chloropentan-2-olarrow_forwardIn the answer box, type the number of maximum stereoisomers possible for the following compound. A H H COH OH = H C Br H.C OH CHarrow_forwardSelect the major product of the following reaction. Br Br₂, light D Br Br Br Brarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning



