
Concept explainers
a)
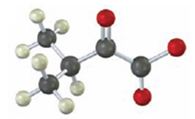
Interpretation:
To identify the amino acid that is a catabolic precursor of the given α-keto acids:
Answer to Problem 17VC
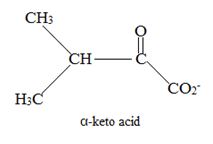
Explanation of Solution
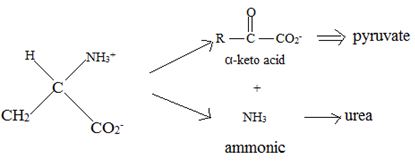
Catabolism of proteins occurs by a discrimination of α – amino acids according to the following reaction:
The retro symmetric strategy is used to derive synthons and starting materials.
b)
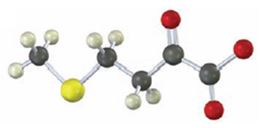
Interpretation:
To identify the amino acid that is a catabolic precursor of the given α-keto acids:
Answer to Problem 17VC
The corresponding α amino acid precursor is respectively:
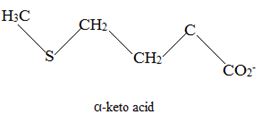
Explanation of Solution
Catabolism of proteins occurs by a discrimination of α – amino acids according to the following reaction:
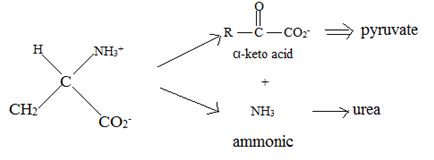
Thus, the α – keto acid derives its structure from its precursor parent amino acid by replacing the 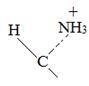 segment by
segment by 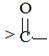 .
.
This proves the above assigned structures for the α – amino acids.
The retro symmetric strategy is used to derive synthons and starting materials.
Want to see more full solutions like this?
Chapter 29 Solutions
EBK ORGANIC CHEMISTRY
- What are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardPlease provide the complete mechanism for the reaction below and include all appropriate arrows, formal charges, and intermediates. Please draw out the answerarrow_forwardPredict the major organic product for this reaction.arrow_forward
- help me with the rf value i am so confusedarrow_forwardPredict the major organic product for this reaction.arrow_forward3) The following molecule, chloral is a common precursor to chloral hydrate, an acetal type molecule that was a first-generation anesthetic. Draw a mechanism that accounts for tis formation and speculate why it does not require the use of an acid catalyst, like most hemiacetal and acetal reaction: (10 pts) H H₂Oarrow_forward
- You are a Quality Manager for a very well-known food ingredient company that produces umami powder, and you are responsible for setting specification limits. The net weight (in grams) of bags of unami powder is monitored by taking samples of six bags on an hourly basis during production. The label on every bag reports a contents of 1KG umami powder. The process mean is μ = 1012 g, and when the process is properly adjusted, it varies with σ = 11 g. QUESTION: Your organisation strives to ensure that >99.97% of bags of umami powder produced conforms to specification. What performance process index value is required to achieve this process yield? Calculate PPK using the following formula: Ppk = (USL – mean)/3 σ Ppk = (mean -LSL)/ 3 σarrow_forwardYou are a Quality Manager for a very well-known food ingredient company that produces umami powder, and you are responsible for setting specification limits. The net weight (in grams) of bags of unami powder is monitored by taking samples of six bags on an hourly basis during production. The label on every bag reports a contents of 1KG umami powder. The process mean is μ = 1012 g, and when the process is properly adjusted, it varies with σ = 11 g. QUESTION: Provide a valid and full justification as to whether you would advise your manager that the process is satisfactory when it is properly adjusted, or would you seek their approval to improve the process?arrow_forwardYou are a Quality Manager for a very well-known food ingredient company that produces umami powder, and you are responsible for setting specification limits. The net weight (in grams) of bags of unami powder is monitored by taking samples of six bags on an hourly basis during production. The label on every bag reports a contents of 1KG umami powder. The process mean is μ = 1012 g, and when the process is properly adjusted, it varies with σ = 11 g. QUESTION: Using all the available information, set the upper and lower specification limits.arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning






