
Concept explainers
Draw the organic product formed when the amino acid leucine is treated with each reagent.
a.
b.
c.
d.
e.
f.
(a)
Interpretation: The organic product formed by the treatment of amino acid leucine with the given reagent is to be drawn.
Concept introduction: The chemical compounds in which carbon is bonded with acidic and basic group along with hydrocarbon side chain are known as amino acids. The amino acids are classified into acidic, basic, polar, non-polar, essential, and non-essential categories. The three word abbreviation or one word abbreviation is used for amino acids.
Answer to Problem 29.40P
The organic product formed by the treatment of amino acid leucine with the given reagent is drawn below.
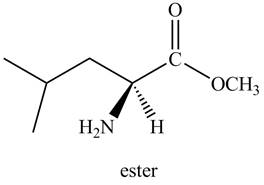
Explanation of Solution
The given reagent is
The reaction between leucine and
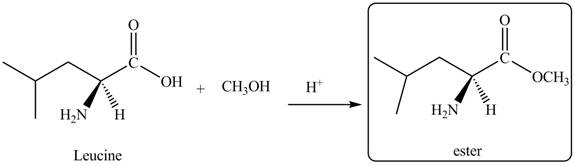
Figure 1
Thus, the organic product formed by the treatment of amino acid leucine with the given reagent is,
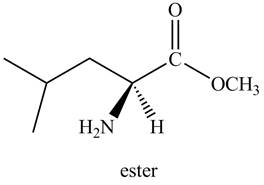
Figure 2
The organic product formed by the treatment of amino acid leucine with the given reagent is drawn in Figure 2.
(b)
Interpretation: The organic product formed by the treatment of amino acid leucine with the given reagent is to be drawn.
Concept introduction: The chemical compounds in which carbon is bonded with acidic and basic group along with hydrocarbon side chain are known as amino acids. The amino acids are classified into acidic, basic, polar, non-polar, essential, and non-essential categories. The three word abbreviation or one word abbreviation is used for amino acids.
Answer to Problem 29.40P
The organic product formed by the treatment of amino acid leucine with the given reagent is drawn below.
Explanation of Solution
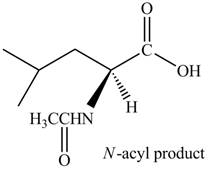
The given reagent is
The treatment of leucine with
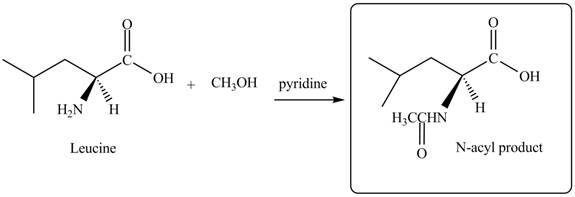
Figure 3
Thus, the organic product formed by the treatment of amino acid leucine with the given reagent is,
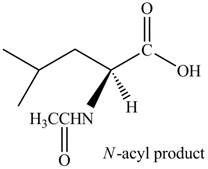
Figure 4
The organic product formed by the treatment of amino acid leucine with the given reagent is drawn in Figure 4.
(c)
Interpretation: The organic product formed by the treatment of amino acid leucine with the given reagent is to be drawn.
Concept introduction: The chemical compounds in which carbon is bonded with acidic and basic group along with hydrocarbon side chain are known as amino acids. The amino acids are classified into acidic, basic, polar, non-polar, essential, and non-essential categories. The three word abbreviation or one word abbreviation is used for amino acids.
Answer to Problem 29.40P
The organic product formed by the treatment of amino acid leucine with the given reagent is drawn below.
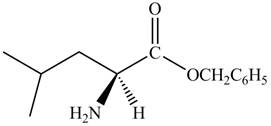
Explanation of Solution
The given reagent is
The reaction between leucine and
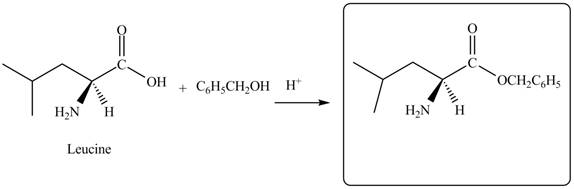
Figure 5
Thus, the organic product formed by the treatment of amino acid leucine with the given reagent is,
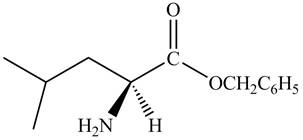
Figure 6
The organic product formed by the treatment of amino acid leucine with the given reagent is drawn in Figure 6.
(d)
Interpretation: The organic product formed by the treatment of amino acid leucine with the given reagent is to be drawn.
Concept introduction: The chemical compounds in which carbon is bonded with acidic and basic group along with hydrocarbon side chain are known as amino acids. The amino acids are classified into acidic, basic, polar, non-polar, essential, and non-essential categories. The three word abbreviation or one word abbreviation is used for amino acids.
Answer to Problem 29.40P
The organic product formed by the treatment of amino acid leucine with the given reagent is drawn below.
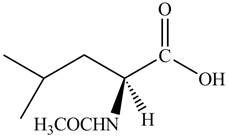
Explanation of Solution
The given reagent is
The reaction between leucine and
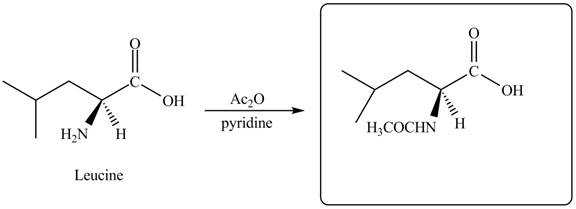
Figure 7
Thus, the organic product formed by the treatment of amino acid leucine with the given reagent is,
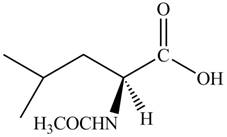
Figure 8
The organic product formed by the treatment of amino acid leucine with the given reagent is drawn in Figure 8.
(e)
Interpretation: The organic product formed by the treatment of amino acid leucine with the given reagent is to be drawn.
Concept introduction: The chemical compounds in which carbon is bonded with acidic and basic group along with hydrocarbon side chain are known as amino acids. The amino acids are classified into acidic, basic, polar, non-polar, essential, and non-essential categories. The three word abbreviation or one word abbreviation is used for amino acids.
Answer to Problem 29.40P
The organic product formed by the treatment of amino acid leucine with the given reagent is drawn below.
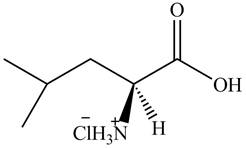
Explanation of Solution
The given reagent is
The reaction between leucine and
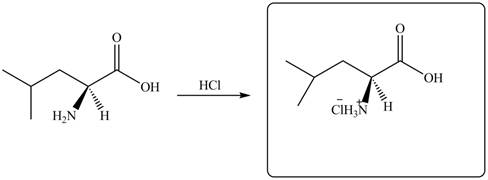
Figure 9
Thus, the organic product formed by the treatment of amino acid leucine with the given reagent is,

Figure 10
The organic product formed by the treatment of amino acid leucine with the given reagent is drawn in Figure 10.
(f)
Interpretation: The organic product formed by the treatment of amino acid leucine with the given reagent is to be drawn.
Concept introduction: The chemical compounds in which carbon is bonded with acidic and basic group along with hydrocarbon side chain are known as amino acids. The amino acids are classified into acidic, basic, polar, non-polar, essential, and non-essential categories. The three word abbreviation or one word abbreviation is used for amino acids.
Answer to Problem 29.40P
The organic product formed by the treatment of amino acid leucine with the given reagent is drawn below.
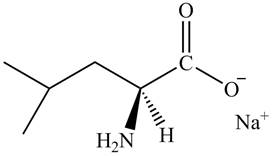
Explanation of Solution
The given reagent is
The reaction between leucine and
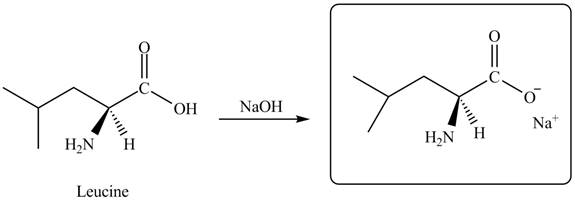
Figure 11
Thus, the organic product formed by the treatment of amino acid leucine with the given reagent is,

Figure 12
The organic product formed by the treatment of amino acid leucine with the given reagent is drawn in Figure 12.
(g)
Interpretation: The organic product formed by the treatment of amino acid leucine with the given reagent is to be drawn.
Concept introduction: The chemical compounds in which carbon is bonded with acidic and basic group along with hydrocarbon side chain are known as amino acids. The amino acids are classified into acidic, basic, polar, non-polar, essential, and non-essential categories. The three word abbreviation or one word abbreviation is used for amino acids.
Answer to Problem 29.40P
The organic product formed by the treatment of amino acid leucine with the given reagent is drawn below.
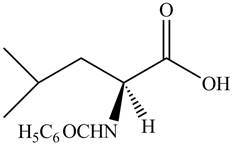
Explanation of Solution
The given reagent is
The reaction between leucine and
Figure 13
Thus, the organic product formed by the treatment of amino acid leucine with the given reagent is,
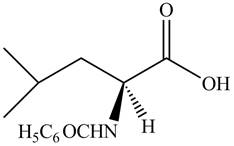
Figure 14
The organic product formed by the treatment of amino acid leucine with the given reagent is drawn in Figure 14.
(h)
Interpretation: The organic product formed by the treatment of amino acid leucine with the given reagent is to be drawn.
Concept introduction: The chemical compounds in which carbon is bonded with acidic and basic group along with hydrocarbon side chain are known as amino acids. The amino acids are classified into acidic, basic, polar, non-polar, essential, and non-essential categories. The three word abbreviation or one word abbreviation is used for amino acids.
Answer to Problem 29.40P
The organic product formed by the treatment of amino acid leucine with the given reagent is drawn below.
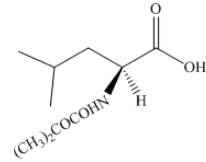
Explanation of Solution
The given reagent is
The reaction between leucine and
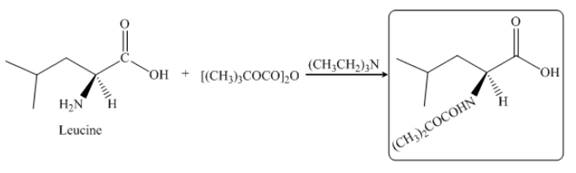
Figure 15
Thus, the organic product formed by the treatment of amino acid leucine with the given reagent is,

Figure 16
The organic product formed by the treatment of amino acid leucine with the given reagent is drawn in Figure 16.
(i)
Interpretation: The organic product formed by the treatment of amino acid leucine with the given reagent is to be drawn.
Concept introduction: The chemical compounds in which carbon is bonded with acidic and basic group along with hydrocarbon side chain are known as amino acids. The amino acids are classified into acidic, basic, polar, non-polar, essential, and non-essential categories. The three word abbreviation or one word abbreviation is used for amino acids.
Answer to Problem 29.40P
The organic product formed by the treatment of amino acid leucine with the given reagent is drawn below.
Explanation of Solution
The given reagent is
The structure of product in (d) is shown below.
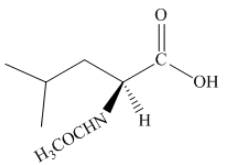
Figure 8
The reaction between leucine and
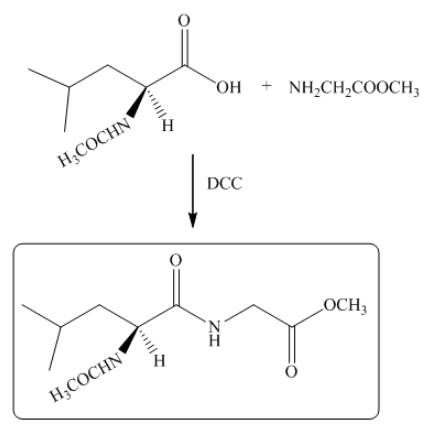
Figure 17
Thus, the organic product formed by the treatment of amino acid leucine with the given reagent is,

Figure 18
The organic product formed by the treatment of amino acid leucine with the given reagent is drawn in Figure 18.
(j)
Interpretation: The organic product formed by the treatment of amino acid leucine with the given reagent is to be drawn.
Concept introduction: The chemical compounds in which carbon is bonded with acidic and basic group along with hydrocarbon side chain are known as amino acids. The amino acids are classified into acidic, basic, polar, non-polar, essential, and non-essential categories. The three word abbreviation or one word abbreviation is used for amino acids.
Answer to Problem 29.40P
The organic product formed by the treatment of amino acid leucine with the given reagent is drawn below.
Explanation of Solution
The given reagent is
The structure of product in (h) is shown below.
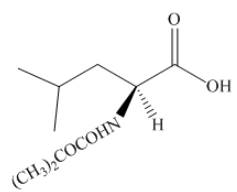
Figure 16
The reaction between leucine and
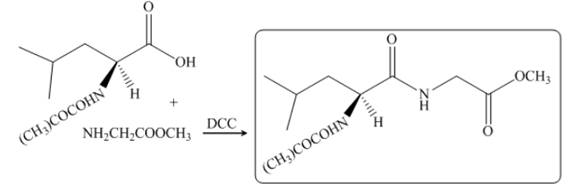
Figure 19
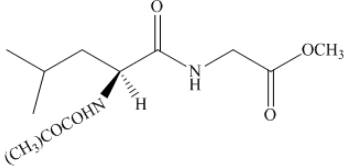
Thus, the organic product formed by the treatment of amino acid leucine with the given reagent is,
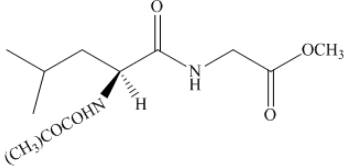
Figure 20
The organic product formed by the treatment of amino acid leucine with the given reagent is drawn in Figure 20.
(k)
Interpretation: The organic product formed by the treatment of amino acid leucine with the given reagent is to be drawn.
Concept introduction: The chemical compounds in which carbon is bonded with acidic and basic group along with hydrocarbon side chain are known as amino acids. The amino acids are classified into acidic, basic, polar, non-polar, essential, and non-essential categories. The three word abbreviation or one word abbreviation is used for amino acids.
Answer to Problem 29.40P
The organic product formed by the treatment of amino acid leucine with the given reagent is drawn below.
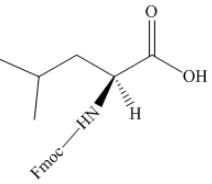
Explanation of Solution
The given reagent is
The reaction between leucine and
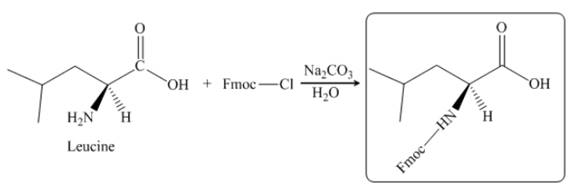
Figure 21
Thus, the organic product formed by the treatment of amino acid leucine with the given reagent is,

Figure 22
The organic product formed by the treatment of amino acid leucine with the given reagent is drawn in Figure 22.
(l)
Interpretation: The organic product formed by the treatment of amino acid leucine with the given reagent is to be drawn.
Concept introduction: The chemical compounds in which carbon is bonded with acidic and basic group along with hydrocarbon side chain are known as amino acids. The amino acids are classified into acidic, basic, polar, non-polar, essential, and non-essential categories. The three word abbreviation or one word abbreviation is used for amino acids.
Answer to Problem 29.40P
The organic product formed by the treatment of amino acid leucine with the given reagent is drawn below.
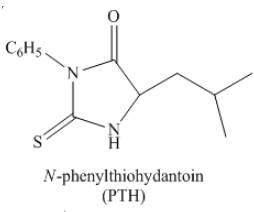
Explanation of Solution
The given reagent is
The reaction between leucine and
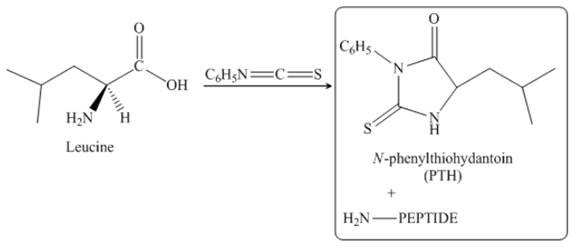
Figure 23
Thus, the organic product formed by the treatment of amino acid leucine with the given reagent is,
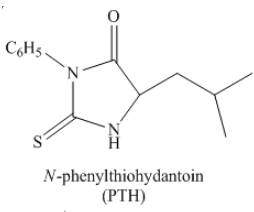
Figure 24
The organic product formed by the treatment of amino acid leucine with the given reagent is drawn in Figure 24.
Want to see more full solutions like this?
Chapter 29 Solutions
Organic Chemistry
- The acid-base chemistry of both EDTA and EBT are important to ensuring that the reactions proceed as desired, thus the pH is controlled using a buffer. What percent of the EBT indicator will be in the desired HIn2- state at pH = 10.5. pKa1 = 6.2 and pKa2 = 11.6 of EBTarrow_forwardCUE COLUMN NOTES (A. Determine Stereoisomers it has ⑤ Identify any meso B compounds cl Br cl -c-c-c-c-¿- 1 CI C- | 2,4-Dichloro-3-bromopentanearrow_forwardThe acid-base chemistry of both EDTA and EBT are important to ensuring that the reactions proceed as desired, thus the pH is controlled using a buffer. What percent of the EBT indicator will be in the desired HIn2- state at pH = 10.5. pKa1 = 6.2 and pKa2 = 11.6 of EBTarrow_forward
- What does the phrase 'fit for purpose' mean in relation to analytical chemistry? Please provide examples too.arrow_forwardFor each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Molecule Inductive Effects Resonance Effects Overall Electron-Density × NO2 ○ donating O donating O withdrawing O withdrawing O electron-rich electron-deficient no inductive effects O no resonance effects O similar to benzene E [ CI O donating withdrawing O no inductive effects Explanation Check ○ donating withdrawing no resonance effects electron-rich electron-deficient O similar to benzene © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accesarrow_forwardUnderstanding how substituents activate Rank each of the following substituted benzene molecules in order of which will react fastest (1) to slowest (4) by electrophilic aromatic substitution. Explanation HN NH2 Check X (Choose one) (Choose one) (Choose one) (Choose one) © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Aarrow_forward
- Identifying electron-donating and electron-withdrawing effects on benzene For each of the substituted benzene molecules below, determine the inductive and resonance effects the substituent will have on the benzene ring, as well as the overall electron-density of the ring compared to unsubstituted benzene. Inductive Effects Resonance Effects Overall Electron-Density Molecule CF3 O donating O donating O withdrawing O withdrawing O no inductive effects O no resonance effects electron-rich electron-deficient O similar to benzene CH3 O donating O withdrawing O no inductive effects O donating O withdrawing Ono resonance effects O electron-rich O electron-deficient O similar to benzene Explanation Check Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forward* Hint: Think back to Chem 1 solubility rules. Follow Up Questions for Part B 12. What impact do the following disturbances to a system at equilibrium have on k, the rate constant for the forward reaction? Explain. (4 pts) a) Changing the concentration of a reactant or product. (2 pts) b) Changing the temperature of an exothermic reaction. (2 pts) ofarrow_forwardDraw TWO general chemical equation to prepare Symmetrical and non-Symmetrical ethers Draw 1 chemical reaction of an etherarrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





