
Organic Chemistry
5th Edition
ISBN: 9780078021558
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 29, Problem 29.39P
Draw the organic products formed in each reaction.
a. 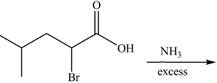 c.
c. 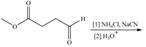
b. 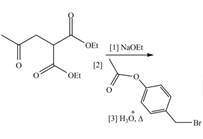 d.
d. 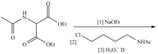
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
2. Specify the solvent and reagent(s) required to carry out each of the following FGI. If
two reagent sets must be used for the FGI, specify the solvent and reagent(s) for each
reagent set. If a reaction cannot be carried out with reagents (sets)
class, write NP (not possible) in the solvent box for reagent set #1.
Use the letter abbreviation for each solvent; use a number abbreviation for reagent(s).
Solvents: CH2Cl2 (A);
H₂O (B);
Reagents:
HBr (1);
R₂BH (6);
H2SO4 (2);
CH3OH (C);
Br₂ (3);
CH3CO₂H (D)
NaHCO3 (4);
Hg(OAc)2 (5);
H₂O2/HO (7);
NaBH4 (8)
Reagent Set #1
Reagent Set #2
FGI
+ enant
OH
Solvent Reagent(s) Solvent Reagent(s)
Germanium (Ge) is a semiconductor with a bandgap of 2.2 eV. How could you dope Ge to make it a p-type semiconductor with a larger bandgap?
Group of answer choices
It is impossible to dope Ge and have this result in a larger bandgap.
Dope the Ge with silicon (Si)
Dope the Ge with gallium (Ga)
Dope the Ge with phosphorus (P)
Which of the following semiconductors would you choose to have photons with the longest possible wavelengths be able to promote electrons to the semiconductor's conduction band?
Group of answer choices
Si
Ge
InSb
CdS
Chapter 29 Solutions
Organic Chemistry
Ch. 29 - Prob. 29.1PCh. 29 - Problem 29.2
What form exists at the isoelectric...Ch. 29 - Problem 29.3
Explain why the of the group of an...Ch. 29 - Prob. 29.4PCh. 29 - Problem 29.5
What -halo carbonyl compound is...Ch. 29 - Problem 29.6
The enolate derived from diethyl...Ch. 29 - Problem 29.7
What amino acid is formed when is...Ch. 29 - Problem 29.8
What aldehyde is needed to synthesize...Ch. 29 - Problem 29.9
Draw the products of each...Ch. 29 - Prob. 29.10P
Ch. 29 - Prob. 29.11PCh. 29 - Prob. 29.12PCh. 29 - Problem 29.13
What alkene is needed to synthesize...Ch. 29 - Problem 29.14
Draw the structure of each peptide....Ch. 29 - Problem 29.15
Name each peptide using both the...Ch. 29 - Prob. 29.16PCh. 29 - Prob. 29.17PCh. 29 - Problem 29.18
Glutathione, a powerful antioxidant...Ch. 29 - Problem 29.19
Draw the structure of the...Ch. 29 - Problem 29.20
Give the amino acid sequence of an...Ch. 29 - a What products are formed when each peptide is...Ch. 29 - Prob. 29.22PCh. 29 - Devise a synthesis of each peptide from amino acid...Ch. 29 - Devise a synthesis of the following dipeptide from...Ch. 29 - Prob. 29.25PCh. 29 - Consider two molecules of a tetrapeptide composed...Ch. 29 - What types of stabilizing interactions exist...Ch. 29 - Prob. 29.28PCh. 29 - Draw the product formed when the following amino...Ch. 29 - With reference to the following peptide: a...Ch. 29 - Devise a synthesis of the following dipeptide from...Ch. 29 - Prob. 29.32PCh. 29 - Histidine is classified as a basic amino acid...Ch. 29 - Tryptophan is not classified as a basic amino acid...Ch. 29 - What is the structure of each amino acid at its...Ch. 29 - What is the predominant form of each of the...Ch. 29 - 29.37 What is the predominant form of each of the...Ch. 29 - a. Draw the structure of the tripeptide A–A–A, and...Ch. 29 - 29.39 Draw the organic products formed in each...Ch. 29 - 29.40 What alkyl halide is needed to synthesize...Ch. 29 - 29.41 Devise a synthesis of threonine from diethyl...Ch. 29 - 29.42 Devise a synthesis of each amino acid from...Ch. 29 - Prob. 29.43PCh. 29 - Prob. 29.44PCh. 29 - Prob. 29.45PCh. 29 - Prob. 29.46PCh. 29 - Prob. 29.47PCh. 29 - 29.48 Brucine is a poisonous alkaloid obtained...Ch. 29 - Prob. 29.49PCh. 29 - Prob. 29.50PCh. 29 - Draw the structure for each peptide: (a) Phe–Ala;...Ch. 29 - 29.52 For the tetrapeptide Asp–Arg–Val–Tyr:
a....Ch. 29 - Prob. 29.53PCh. 29 - Prob. 29.54PCh. 29 - 29.55 Draw the amino acids and peptide fragments...Ch. 29 - Prob. 29.56PCh. 29 - Prob. 29.57PCh. 29 - Prob. 29.58PCh. 29 - 29.59 An octapeptide contains the following amino...Ch. 29 - 29.60 Draw the organic products formed in each...Ch. 29 - Draw all the steps in the synthesis of each...Ch. 29 - 29.62 Write out the steps for the synthesis of...Ch. 29 - 29.63 Besides the Boc and Fmoc protecting groups...Ch. 29 - 29.64 Another method to form a peptide bond...Ch. 29 - 29.65 Draw the mechanism for the reaction that...Ch. 29 - 29.66 Which of the following amino acids are...Ch. 29 - 29.67 After the peptide chain of collagen has been...Ch. 29 - Prob. 29.68PCh. 29 - Prob. 29.69PCh. 29 - 29.70 The anti-obesity drug orlistat works by...Ch. 29 - Prob. 29.71P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Some people consider Pasteur or Koch to be the Father of Microbiology, rather than Leeuwenhoek. Why might they ...
Microbiology with Diseases by Body System (5th Edition)
Gregor Mendel never saw a gene, yet he concluded that some inherited factors were responsible for the patterns ...
Campbell Essential Biology (7th Edition)
To test your knowledge, discuss the following topics with a study partner or in writing ideally from memory. Th...
HUMAN ANATOMY
Choose the best answer to each of the following. Explain your reasoning. If Earth were twice as far as it actua...
Cosmic Perspective Fundamentals
Whether two metal foil leaves an electroscope get opposite charge when the electroscope is charged.
Physics of Everyday Phenomena
More than one choice may apply. Using the terms listed below, fill in the blank with the proper term. anterior ...
Essentials of Human Anatomy & Physiology (12th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following metals is the only one with all of its bands completely full? Group of answer choices K Na Ca Alarrow_forward2. Specify the solvent and reagent(s) required to carry out each of the following FGI. If two reagent sets must be used for the FGI, specify the solvent and reagent(s) for each reagent set. If a reaction cannot be carried out with reagents (sets) class, write NP (not possible) in the solvent box for reagent set #1. Use the letter abbreviation for each solvent; use a number abbreviation for reagent(s). Solvents: CH2Cl2 (A); Reagents: H₂O (B); CH3CO₂H (D) NaHCO3 (4); Hg(OAc)2 (5); HBr (1); R₂BH (6); H2SO4 (2); CH3OH (C); Br₂ (3); H₂O₂ / HO- (7); NaBH4 (8) Reagent Set #1 Reagent Set #2 FGI OH - α-α Br + enant Solvent Reagent(s) Solvent Reagent(s)arrow_forwardBased on concepts from Lecture 3-5, which of the following ionic compounds should be most soluble in water? Group of answer choices MgO BeO CaO BaOarrow_forward
- From an energy standpoint, which two process - in the correct order - are involved in the dissolving of an ionic compound crystal? Group of answer choices Water coordination to the ions followed by sublimation into the gas phase Sublimation of the crystal into gas-phase ions followed by water coordination to the ions Ion dissociation from the crystal followed by water coordination to the ions Water coordination to the ions followed by ion dissociation from the crystalarrow_forwardFor which Group 2 metal (M), is this process the most exothermic? M2+(g) + O2−(g) + CO2(g) → MO(s) + CO2(g) Group of answer choices M = Sr M = Mg M = Ca M = Baarrow_forward2. Specify the solvent and reagent(s) required to carry out each of the following FGI. If two reagent sets must be used for the FGI, specify the solvent and reagent(s) for each reagent set. If a reaction cannot be carried out with reagents (sets) class, write NP (not possible) in the solvent box for reagent set #1. Use the letter abbreviation for each solvent; use a number abbreviation for reagent(s). Solvents: CH2Cl2 (A); H₂O (B); Reagents: HBr (1); H2SO4 (2); CH3OH (C); Br₂ (3); CH3CO₂H (D) NaHCO3 (4); Hg(OAc)2 (5); R₂BH (6); H₂O₂ / HO- (7); NaBH4 (8) Reagent Set #1 Reagent Set #2 FGI Solvent Reagent(s) Solvent Reagent(s) HO OHarrow_forward
- For which of the following ionic compounds would you expect the smallest difference between its theoretical and experimental lattice enthalpies? (You may assume these all have the same unit cell structure.) Electronegativities: Ca (1.0), Fe (1.8), Mg (1.2), O (3.5), S (2.5), Zn (1.6) Group of answer choices ZnO MgS CaO FeSarrow_forwardIn the Born-Haber cycle for KCl crystal formation, what enthalpy component must be divided by two? Group of answer choices KCl(s) enthalpy of formation Ionization energy for K(g) K(s) sublimation enthalpy Cl2 bond dissociation enthalpyarrow_forward2. Specify the solvent and reagent(s) required to carry out each of the following FGI. If two reagent sets must be used for the FGI, specify the solvent and reagent(s) for each reagent set. If a reaction cannot be carried out with reagents (sets) class, write NP (not possible) in the solvent box for reagent set #1. Use the letter abbreviation for each solvent; use a number abbreviation for reagent(s). Solvents: CH2Cl2 (A); H₂O (B); Reagents: HBr (1); R₂BH (6); H2SO4 (2); CH3OH (C); Br₂ (3); CH3CO₂H (D) NaHCO3 (4); Hg(OAc)2 (5); H₂O₂ / HO (7); NaBH4 (8) Reagent Set #1 Reagent Set #2 FGI хот Br Solvent Reagent(s) Solvent Reagent(s)arrow_forward
- What is the correct chemical equation for the lattice formation reaction for CaBr2? Group of answer choices Ca2+(g) + 2 Br−(g) → CaBr2(s) ½ Ca2+(g) + Br−(g) → ½ CaBr2(s) Ca(s) + Br2(l) → CaBr2(s) Ca(s) + 2 Br−(g) → CaBr2(s)arrow_forwardPLEASE ANSWER THE QUESTION!!!arrow_forward3. SYNTHESIS. Propose a sequence of synthetic steps (FGI) that convert the starting material (SM) into the Target molecule. For each FGI in your proposed synthesis, specify the reagents / conditions, and draw the product(s) of that FGI. DO NOT INCLUDE the FGI mxn in the answer you submit. If an FGI requires two reagent sets, specify the order in which the reagent sets are added, e.g., i) Hg(OAc)2 / H₂O; ii) NaBH4/MeOH. Indicate the stereochemistry (if any) of the products of each FGI. FGI 1. Me Starting Material Source of all carbons in the Target molecule (can use multiple copies) Me Me Target molecule + enantiomerarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY