
(a)
Interpretation:
It should be identified that will a thermal 1, 3-migrations of carbon occur with whether retention or inversion of configuration.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Electrocyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
In a sigmatropic reaction “ one new sigma-bond is formed as another breaks.”
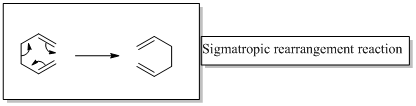
Sigmatropic rearrangement reactions are named with digits. For example a [1, 3] sigmatropic rearrangement describe a reaction in which the residue migrates from position 1 to position 3.
Migration of carbon and hydrogen will occur in a sigmatropic rearrangement reaction.
When hydrogen migrates in a sigmatropic rearrangement, the s orbital of the hydrogen is partially bonded to both the migration origin and the migration terminus in the transition state.
Migration of hydrogen in suprafacial and antarafacial rearrangement can be represented as follows,


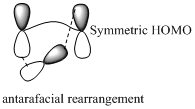
Migration of carbon occurs through two ways because it has a two lobed p orbital. Carbon can simultaneously interact with the migration origin and the migration terminus using one lobe of its p orbital.
Migration of carbon in suprafacial and antarafacial rearrangement can be represented as follows,
Carbon migrating with one lobe of its p orbital interacting


Carbon migrating with both lobe of its p orbital interacting

Inversion of configuration is the inversion of a chiral center in a molecule in a
Retention of configuration is the preservation of integrity of the spatial arrangement of bonds to a chiral center during a chemical reaction.
(b)
Interpretation:
It should be identified that will a thermal 1, 5-migrations of carbon occur with whether retention or inversion of configuration.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Electrocyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
In a sigmatropic reaction “ one new sigma-bond is formed as another breaks.”

Sigmatropic rearrangement reactions are named with digits. For example a [1, 3] sigmatropic rearrangement describe a reaction in which the residue migrates from position 1 to position 3.
Migration of carbon and hydrogen will occur in a sigmatropic rearrangement reaction.
When hydrogen migrates in a sigmatropic rearrangement, the s orbital of the hydrogen is partially bonded to both the migration origin and the migration terminus in the transition state.
Migration of hydrogen in suprafacial and antarafacial rearrangement can be represented as follows,


Migration of carbon occurs through two ways because it has a two lobed p orbital. Carbon can simultaneously interact with the migration origin and the migration terminus using one lobe of its p orbital.
Migration of carbon in suprafacial and antarafacial rearrangement can be represented as follows,
Carbon migrating with one lobe of its p orbital interacting


Carbon migrating with both lobe of its p orbital interacting


Inversion of configuration is the inversion of a chiral center in a molecule in a chemical reaction.
Retention of configuration is the preservation of integrity of the spatial arrangement of bonds to a chiral center during a chemical reaction.
Want to see the full answer?
Check out a sample textbook solution
Chapter 28 Solutions
Organic Chemistry Study Guide and Solutions Manual, Books a la Carte Edition (8th Edition)
- Don't used hand raiting and don't used Ai solutionarrow_forwardHighlight in red each acidic location on the organic molecule at left. Highlight in blue each basic location on the organic molecule at right. Note for advanced students: we mean acidic or basic in the Brønsted-Lowry sense only. Cl N شیخ x Garrow_forwardQ4: Draw the mirror image of the following molecules. Are the molecules chiral? C/ F LL CI CH3 CI CH3 0 CI CH3 CI CH3 CH3arrow_forward
- Complete combustion of a 0.6250 g sample of the unknown crystal with excess O2 produced 1.8546 g of CO2 and 0.5243 g of H2O. A separate analysis of a 0.8500 g sample of the blue crystal was found to produce 0.0465 g NH3. The molar mass of the substance was found to be about 310 g/mol. What is the molecular formula of the unknown crystal?arrow_forward4. C6H100 5 I peak 3 2 PPM Integration values: 1.79ppm (2), 4.43ppm (1.33) Ipeakarrow_forwardNonearrow_forward
- 3. Consider the compounds below and determine if they are aromatic, antiaromatic, or non-aromatic. In case of aromatic or anti-aromatic, please indicate number of I electrons in the respective systems. (Hint: 1. Not all lone pair electrons were explicitly drawn and you should be able to tell that the bonding electrons and lone pair electrons should reside in which hybridized atomic orbital 2. You should consider ring strain- flexibility and steric repulsion that facilitates adoption of aromaticity or avoidance of anti- aromaticity) H H N N: NH2 N Aromaticity (Circle) Aromatic Aromatic Aromatic Aromatic Aromatic Antiaromatic Antiaromatic Antiaromatic Antiaromatic Antiaromatic nonaromatic nonaromatic nonaromatic nonaromatic nonaromatic aromatic TT electrons Me H Me Aromaticity (Circle) Aromatic Aromatic Aromatic Aromatic Aromatic Antiaromatic Antiaromatic Antiaromatic Antiaromatic Antiaromatic nonaromatic nonaromatic nonaromatic nonaromatic nonaromatic aromatic πT electrons H HH…arrow_forwardA chemistry graduate student is studying the rate of this reaction: 2 HI (g) →H2(g) +12(g) She fills a reaction vessel with HI and measures its concentration as the reaction proceeds: time (minutes) [IH] 0 0.800M 1.0 0.301 M 2.0 0.185 M 3.0 0.134M 4.0 0.105 M Use this data to answer the following questions. Write the rate law for this reaction. rate = 0 Calculate the value of the rate constant k. k = Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol.arrow_forwardNonearrow_forward
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning

