
(a)
Interpretation: The product of given reaction has to be drawn.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Electrocyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
In an electrocyclic reaction “one new sigma- bond is formed or brocken.”
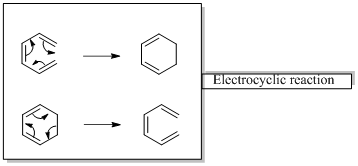
Woodward –Hoffmann rules are the set of rules used to vindicate or predict certain aspects of the stereo chemical outcome and activation energy of pericyclic reactions.
Woodward – Hoffmann rules for Electrocyclic reactions are listed below
A photochemical reaction takes place when a reactant absorbs light and a thermal reaction takes place without the absorption of light.
Woodward – Hoffmann rules for the configuration of electrocyclic reactions are,
(b)
Interpretation: The product of given reaction has to be drawn.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Electrocyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
In an electrocyclic reaction “one new sigma- bond is formed or brocken.”

Woodward –Hoffmann rules are the set of rules used to vindicate or predict certain aspects of the stereo chemical outcome and activation energy of pericyclic reactions.
Woodward – Hoffmann rules for Electrocyclic reactions are listed below
A photochemical reaction takes place when a reactant absorbs light and a thermal reaction takes place without the absorption of light.
Woodward – Hoffmann rules for the configuration of electrocyclic reactions are,
Want to see the full answer?
Check out a sample textbook solution
Chapter 28 Solutions
Organic Chemistry Study Guide and Solutions Manual, Books a la Carte Edition (8th Edition)
- Nonearrow_forward(9 Pts) In one of the two Rare Earth element rows of the periodic table, identify an exception to the general ionization energy (IE) trend. For the two elements involved, answer the following questions. Be sure to cite sources for all physical data that you use. a. (2 pts) Identify the two elements and write their electronic configurations. b. (2 pts) Based on their configurations, propose a reason for the IE trend exception. c. (5 pts) Calculate effective nuclear charges for the last electron in each element and the Allred-Rochow electronegativity values for the two elements. Can any of these values explain the IE trend exception? Explain how (not) - include a description of how IE relates to electronegativity.arrow_forwardPlease explain thoroughly and provide steps to draw.arrow_forward
- As you can see in the picture, the instrument uses a Xe source. Given that the instrument is capable of measuring from 200-800nm, if Xe was not used, what other source(s) could be used? Refer to figure 7-3. How many monochrometers does this instrument have? Why? Trace the light as it goes from the Xenon lamp all the way to the circle just slightly to the right and a little bit down from S4. What do you think that circle is? In class we talked about many types of these, which kind do you think this one is for a fluorimeter? Why? Explain. What is/are some strategy(ies) that this instrument has for dealing with noise that you see present in the optics diagram? Why does a fluorescence cuvette have to be clear on four sides?arrow_forwardProvide steps and thoroughly solve.arrow_forwardNonearrow_forward
- Devise a synthesis to prepare 4-tert-butyl-2-nitrotoluene from toluene. Complete the following reaction scheme. Part 1 of 4 Step 1 Step 2 A B Draw the structure for compound B, 4-tert-butyl-2-nitrotoluene. Click and drag to start drawing a structure. 'O Х ப:arrow_forwardWhat is N hybridized? sp3 or sp2? whyarrow_forwardDate Unknown o Hydrated Salt Lab Sec. Name Trial I Trial 2 1. Mass of fired crucible and lid (g) 2. Mass of fired crucible, lid, and hydrated sah (g) 3. Instructor's approval of flame and apparatus 4. Mass of crucible, lid, and anhydrous salt Ist mass measurement (g) 2nd mass measurement (g) 3rd mass measurement (g). Desk No. Trial 3 48.833 46.808 213.692 51.507 9.359 46,615 50.296 48.211 45.351 50.142 48.146 45.1911 50.103 48.132 45.186 5. Final mass of crucible, lid, and anhydrous salt (g) 50.180 4.13 45.243 Calculations 1. Mass of hydrated salt (g) 2. Mass of anhydrous salt (g) 2.674 2.491 2.9239 1.3479 1.2959 1.5519 3. Mass of water lost (g) 1.32791969 1.322g 4. Percent by mass of volatile water in hydrated salt (%) 49.6% 48% 216.9% 5. Average percent HO in bydrated salt (%H,O) 5. Standard deviation of %H,O Relative standard deviation of %H,O in hydrated salt (RSD) how calculations on next page. 48.17% Data Analysis, B Data Analysis, C Data Analysis, D Experiment 5 89arrow_forward
- Considering the irregular electronic configurations we discussed for certain transitionmetals, think about the possibility of silicon (Si) having a [Ne]3s 2 3p 2 configuration vs.[Ne]3s 1 3p 3. Discuss the pros and cons of both configurations. Which one does Si actuallyadopt and why?arrow_forward(5 Pts) Currently, the last element in the periodic table is number 118, oganesson (Og). Channel your inner Dimitri Mendeleev and predict element 119’s electronic configuration, atomic mass, density, and either melting or boiling point. Justify your answers.arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning



