
Concept explainers
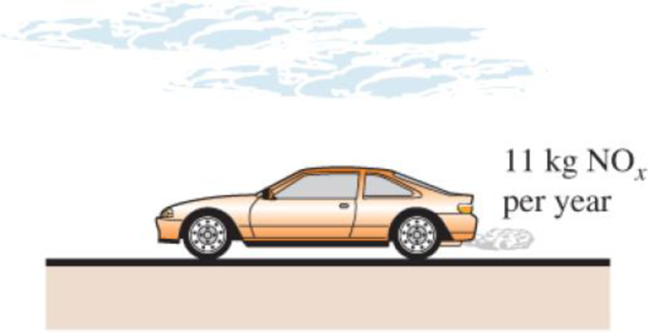
A typical car driven 20,000 km a year emits to the atmosphere about 11 kg per year of NOx (nitrogen oxides), which cause smog in major population areas. Natural gas burned in the furnace emits about 4.3 g of NOx per therm (1 therm = 105,500 kJ), and the electric power plants emit about 7.1 g of NOx per kWh of electricity produced. Consider a household that has two cars and consumes 9000 kWh of electricity and 1200 therms of natural gas per year. Determine the amount of NOx emission to the atmosphere per year for which this household is responsible.
FIGURE P2–83
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
Thermodynamics: An Engineering Approach ( 9th International Edition ) ISBN:9781260092684
Additional Engineering Textbook Solutions
EBK FUNDAMENTALS OF THERMODYNAMICS, ENH
INTERNATIONAL EDITION---Engineering Mechanics: Statics, 14th edition (SI unit)
DESIGN OF MACHINERY
Fundamentals Of Thermodynamics
Fluid Mechanics Fundamentals And Applications
Automotive Technology: Principles, Diagnosis, and Service (5th Edition)
- A steam power station uses coals of calorific value of 6045-kcal/kg. The overall efficiency ofthe power station is 21.5% and a coal power station uses 4.05-tonnes of coal every 15 minutesto generate the maximum demand. The purchasing price of coals is R600 per tonne. (NB:1 kWh = 861 kcal)(a) Determine the coal consumption in kg per kWh.(b) Determine the cost of purchasing the coals per week if the load factor is 65%.arrow_forwardSaturated air at 55F dry bulb (db) temperature is heated to 100 F db. The required heating energy, in BTU/lbair, is most likely: 11 Btu/lbm 15 Btu/lb 9 Btu/lb 5 Btu/lb 20 Btu/lbarrow_forwardA gas is contained in a vertical piston-cylinder assembly by a piston with a face area of 50 in² and weight of 100 lbf. The atmosphere exerts a pressure of 14.7 lb-/in² on top of the piston. A paddle wheel transfers 3 Btu of energy to the gas during a process in which the elevation of the piston increases slowly by 1 ft. The piston and cylinder are poor thermal conductors, and friction between the piston and cylinder can be neglected. Determine the work done by the gas on the piston, in Btu, and the change in internal energy of the gas, in Btu. Step 1 * Your answer is incorrect. Determine the expansion work done by the gas on the piston, in Btu. Wexp Hint 12.876 Save for Later Btu Attempts: 1 of 4 used Submit Answer Step 2 The parts of this question must be completed in order. This part will be available when you complete the part above.arrow_forward
- One way to improve the fuel efficiency of a car is to use tires that have a lower rolling resistance—tires that roll with less resistance. Highway tests at 65 mph showed that tires with the lowest rolling resistance can improve fuel efficiency by nearly 2 mpg (miles per gallon).arrow_forwardi need the answer quicklyarrow_forwardDescribe the heat and work are energy transfer mechanisms between a system and its surroundings, and there are many similarities between them?arrow_forward
- A room is heated by a baseboard resistance heater. When the heat losses from the room on a winter day amount to 9000 kJ/h, it is observed that the air temperature in the room remains constant even though the heater operates continuously. Determine the power rating of the heater, in kW.arrow_forwardA gas is contained in a vertical piston-cylinder assembly by a piston with a face area of 50 in² and weight of 100 lbf. The atmosphere exerts a pressure of 14.7 lb/in² on top of the piston. A paddle wheel transfers 3 Btu of energy to the gas during a process in which the elevation of the piston increases slowly by 2 ft. The piston and cylinder are poor thermal conductors, and friction between the piston and cylinder can be neglected. Determine the work done by the gas on the piston, in Btu, and the change in internal energy of the gas, in Btu. * Your answer is incorrect. Determine the expansion work done by the gas on the piston, in Btu. Wexp = i 0.854 Btuarrow_forwardThe demand for electric power is usually much higher during the day than it is at night, and utility companies often sell power at night at much lower prices to encourage consumers to use the available power generation capacity and to avoid building new expensive power plants that will be used only a short time during peak periods. Utilities are also willing to purchase power produced during the day from private parties at a high price. Suppose a utility company is selling electric power for $0.06/kWh at night and is willing to pay $0.13/kWh for power produced during the day. To take advantage of this opportunity, an entrepreneur is considering building a large reservoir 50 m above the lake level, pumping water from the lake to the reservoir at night using cheap power, and letting the water flow from the reservoir back to the lake during the day, producing power as the pump-motor operates as a turbine-generator during reverse flow. Preliminary analysis shows that a water flow rate of 2…arrow_forward
- The demand for electric power is usually much higher during the day than it is at night, and utility companies often sell power at night at much lower prices to encourage consumers to use the available power generation capacity and to avoid building new expensive power plants that will be used only a short time during peak periods. Utilities are also willing to purchase power produced during the day from private parties at a high price. Suppose a utility company is selling electric power for $0.06/kWh at night and is willing to pay $0.13/kWh for power produced during the day. To take advantage of this opportunity, an entrepreneur is considering building a large reservoir 50 m above the lake level, pumping water from the lake to the reservoir at night using cheap power, and letting the water flow from the reservoir back to the lake during the day, producing power as the pump–motor operates as a turbine–generator during reverse flow. Preliminary analysis shows that a water flow rate of 2…arrow_forwardAn ideal gas (at STP, standard temperature and pressure) occupies a volume of 22.4 liters. While absorbing heat from the surroundings, the gas isobarically expands to 32.4 liters. What is the change in the internal energy of the gas? 1.52 kJ 2.53 kJ O 4.45 kJ 0.75 kJarrow_forwardThermodynamicsarrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





