
Concept explainers
Interpretation:
The two product in the given reaction are formed after [1, 7] sigmatropic rearrangement, one due to hydrogen migration and the other to deuterium migration. It should be represented the configuration of product by replacing the A and B with appropriate atoms (H or D)
Concept introduction:
In a sigmatropic reaction “ one new sigma-bond is formed as another breaks.”
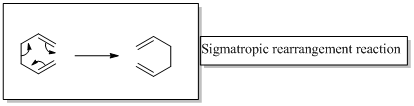
Sigma tropic rearrangement reactions are designated with digits. For example a [1, 3] sigma tropic rearrangement describe a reaction in which the residue migrates from position 1 to position 3
Woodward –Hoffmann rules are the set of rules used to vindicate or predict certain aspects of the stereo chemical outcome and activation energy of pericyclic reactions.
Woodward – Hoffmann rules for sigma tropic rearrangement reactions are listed below
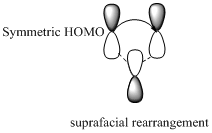
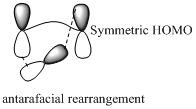
Migration of carbon and hydrogen will occur in a sigmatropic rearrangement reaction. Migration of hydrogen in suprafacial and antarafacial rearrangement can be represented as follows,


Carbon migrating with one lobe of its p orbital interacting

Carbon migrating with both lobe of its p orbital interacting

Want to see the full answer?
Check out a sample textbook solution
Chapter 28 Solutions
Student's Study Guide and Solutions Manual for Organic Chemistry
- Draw the major product of this reaction. Ignore inorganic byproducts. ○ O 1. H₂O, pyridine 2. neutralizing work-up a N W X 人 Parrow_forward✓ Check the box under each molecule that has a total of five ẞ hydrogens. If none of the molecules fit this description, check the box underneath the table. tab OH CI 0 Br xx Br None of these molecules have a total of five ẞ hydrogens. esc Explanation Check caps lock shift 1 fn control 02 F2 W Q A N #3 S 80 F3 E $ t 01 205 % 5 F5 & 7 © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility FT * 8 R T Y U כ F6 9 FIG F11 F D G H J K L C X V B < N M H option command P H + F12 commandarrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts and the carboxylic acid side product. O 1. CHзMgBr (excess) 2. H₂O ✓ W X 人arrow_forward
- If cyclopentyl acetaldehyde reacts with NaOH, state the product (formula).arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. N S S HgCl2, H2SO4 く 8 W X Parrow_forwardtab esc く Drawing the After running various experiments, you determine that the mechanism for the following reaction occurs in a step-wise fashion. Br + OH + Using this information, draw the correct mechanism in the space below. 1 Explanation Check F2 F1 @2 Q W A os lock control option T S # 3 80 F3 Br $ 4 0105 % OH2 + Br Add/Remove step X C F5 F6 6 R E T Y 29 & 7 F D G H Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Ce A F7 DII F8 C Ո 8 * 9 4 F10 F C J K L C V Z X B N M H command P ge Coarrow_forward
- Indicate compound A that must react with ethylbenzene to obtain 4-ethylbenzene-1-sulfonic acid. 3-bromo-4-ethylbenzene-1-sulfonic acid.arrow_forwardPart 1 of 2 Draw the structure of A, the minor E1 product of the reaction. esc I Skip Part Check H₂O, D 2 A + Click and drag to start drawing a structure. -0- F1 F2 1 2 # 3 Q A 80 F3 W E S D F4 $ 4 % 5 F5 ㅇ F6 R T Y F G X 5 & 7 + Save 2025 McGraw Hill LLC. All Rights Reserved. DII F7 F8 H * C 80 J Z X C V B N 4 F9 6arrow_forwardFile Preview The following is a total synthesis of the pheromone of the western pine beetle. Such syntheses are interesting both because of the organic chemistry, and because of the possibility of using species specific insecticides, rather than broad band insecticides. Provide the reagents for each step. There is some chemistry from our most recent chapter in this synthesis, but other steps are review from earlier chapters. (8 points) COOEt COOEt A C COOEt COOEt COOH B OH OTS CN D E See the last homework set F for assistance on this one. H+, H₂O G OH OH The last step is just nucleophilic addition reactions, taking the ketone to an acetal, intramolecularly. But it is hard to visualize the three dimensional shape as it occurs. Frontalin, pheromone of the western pine beetlearrow_forward
- For the reaction below: 1. Draw all reasonable elimination products to the right of the arrow. 2. In the box below the reaction, redraw any product you expect to be a major product. C Major Product: Check + ◎ + X ง © Cl I F2 80 F3 I σ F4 I F5 NaOH Click and drawing F6 A 2025 McGraw Hill LLC. All Rights E F7 F8 $ # % & 2 3 4 5 6 7 8 Q W E R T Y U A S D F G H Jarrow_forwardCan I please get help with this graph. If you can show exactly where it needs to pass through.arrow_forwardN Draw the major product of this reaction. Ignore inorganic byproducts. D 1. H₂O, pyridine 2. neutralizing work-up V P W X DE CO e C Larrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

