
Concept explainers
Draw the product of each of the following reactions:
(a)
Interpretation:
The product formed in the given reaction has to be drawn.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Pericyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
Woodward –Hoffmann rules are the set of rules used to vindicate or predict certain aspects of the stereo chemical outcome and activation energy of pericyclic reactions.
Woodward – Hoffmann rules for pericyclic reactions are listed below
A photochemical reaction takes place when a reactant absorbs light and a thermal reaction takes place without the absorption of light.
Woodward – Hoffmann rules for the configuration of pericyclic reactions are,
Answer to Problem 23P
Product formed in the given reaction is,
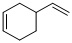
Explanation of Solution
Given reaction is,
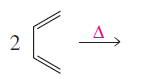
Herein, the compound has an even number of
Therefore the structure of the product formed in the reaction is,
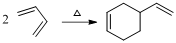
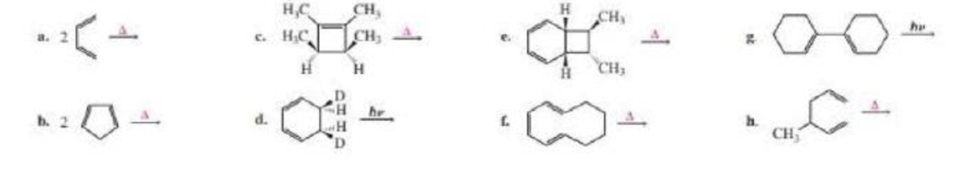
(b)
Interpretation:
The product formed in the given reaction has to be drawn.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Pericyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
Woodward –Hoffmann rules are the set of rules used to vindicate or predict certain aspects of the stereo chemical outcome and activation energy of pericyclic reactions.
Woodward – Hoffmann rules for Pericyclic reactions are listed below
A photochemical reaction takes place when a reactant absorbs light and a thermal reaction takes place without the absorption of light.
Woodward – Hoffmann rules for the configuration of pericyclic reactions are,
Answer to Problem 23P
Product of given reaction is,
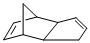
Explanation of Solution
Given reaction is,
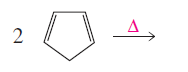
Herein, the compound has an even number of
Therefore the structure of the product formed in the reaction is,
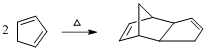
(c)
Interpretation:
The product formed in the given reaction has to be drawn.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Pericyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
Woodward –Hoffmann rules are the set of rules used to vindicate or predict certain aspects of the stereo chemical outcome and activation energy of pericyclic reactions.
Woodward – Hoffmann rules for Pericyclic reactions are listed below
A photochemical reaction takes place when a reactant absorbs light and a thermal reaction takes place without the absorption of light.
Woodward – Hoffmann rules for the configuration of pericyclic reactions are,
Answer to Problem 23P
The product of the given reaction is,
Explanation of Solution
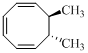
Given reaction is,
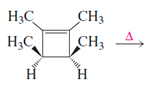
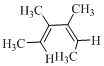
Here in the compound in which the four methyl substituent point in opposite directions, then according to Woodward-Hoffmann rules, they will be “cis” in the ring-closed product when ring closure is conrotatory and “trans” in the ring-closed product when ring closure is disrotatory.
Therefore the structure of the product formed in the reaction is,
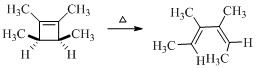
(d)
Interpretation:
The product formed in the given reaction has to be drawn.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Pericyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
Woodward –Hoffmann rules are the set of rules used to vindicate or predict certain aspects of the stereo chemical outcome and activation energy of pericyclic reactions.
Woodward – Hoffmann rules for Pericyclic reactions are listed below
A photochemical reaction takes place when a reactant absorbs light and a thermal reaction takes place without the absorption of light.
Woodward – Hoffmann rules for the configuration of pericyclic reactions are,
Answer to Problem 23P
Product of the given reaction is,
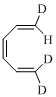
Explanation of Solution
Given reaction is,
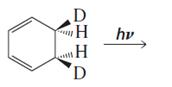
Here in the compound in which the two D and H substituent point in same directions, then according to Woodward-Hoffmann rules, they will be “trans” in the ring-closed product when ring closure is disrotatory and cis in the ring-closed product when ring closure is conrotatory.
Therefore the structure of the product formed in the reaction is,
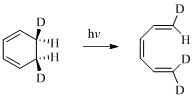
(e)
Interpretation:
The product formed in the given reaction has to be drawn.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Pericyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
Woodward –Hoffmann rules are the set of rules used to vindicate or predict certain aspects of the stereo chemical outcome and activation energy of pericyclic reactions.
Woodward – Hoffmann rules for Pericyclic reactions are listed below
A photochemical reaction takes place when a reactant absorbs light and a thermal reaction takes place without the absorption of light.
Woodward – Hoffmann rules for the configuration of pericyclic reactions are,
Answer to Problem 23P
The product of given reaction is,
Explanation of Solution
Given reaction is,
Here in the compound have two
Therefore the structure of the product formed in the reaction is,
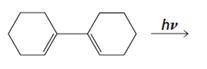
(f)
Interpretation:
The product formed in the given reaction has to be drawn.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Pericyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
Woodward –Hoffmann rules are the set of rules used to vindicate or predict certain aspects of the stereo chemical outcome and activation energy of pericyclic reactions.
Woodward – Hoffmann rules for Pericyclic reactions are listed below
A photochemical reaction takes place when a reactant absorbs light and a thermal reaction takes place without the absorption of light.
Woodward – Hoffmann rules for the configuration of pericyclic reactions are,
Answer to Problem 23P
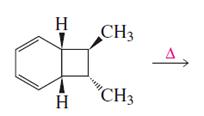
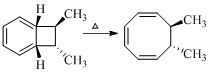
Product of given reaction is,
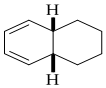
Explanation of Solution
Given reaction is,
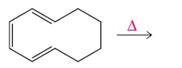
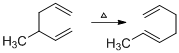
The reaction carried out under thermal conditions then according with the several Woodward - Hoffmann rules the probable structure formed in the given reaction is,
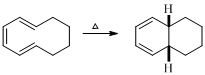
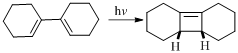
(g)
Interpretation:
The product formed in the given reaction has to be drawn.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Pericyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
Woodward –Hoffmann rules are the set of rules used to vindicate or predict certain aspects of the stereo chemical outcome and activation energy of pericyclic reactions.
Woodward – Hoffmann rules for Pericyclic reactions are listed below
A photochemical reaction takes place when a reactant absorbs light and a thermal reaction takes place without the absorption of light.
Woodward – Hoffmann rules for the configuration of pericyclic reactions are,
Answer to Problem 23P
The product of given reaction is,
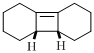
Explanation of Solution
Given reaction is,
Here in the compound have two
(h)
Interpretation:
The product formed in the given reaction has to be drawn.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
There are mainly three types of pericyclic reactions,
- 1) Pericyclic reactions
- 2) Cycloaddition reactions
- 3) Sigmatropic reactions
Woodward –Hoffmann rules are the set of rules used to vindicate or predict certain aspects of the stereo chemical outcome and activation energy of pericyclic reactions.
Woodward – Hoffmann rules for Pericyclic reactions are listed below
A photochemical reaction takes place when a reactant absorbs light and a thermal reaction takes place without the absorption of light.
Woodward – Hoffmann rules for the configuration of pericyclic reactions are,
Answer to Problem 23P
Product of given reaction is,
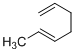
Explanation of Solution
Given reaction is,
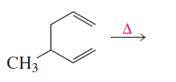
According to the Woodward-Hoffmann rules, the reactant coverts to product under thermal condition and the number of
The structure of the product formed in the reaction is,
Want to see more full solutions like this?
Chapter 28 Solutions
Student's Study Guide and Solutions Manual for Organic Chemistry
- Name the following carbohydrates give both the systematic and common names. Don't forget to identify the Isomer.arrow_forwardWhat is the product of the reaction of XeF4 with H2O? Group of answer choices H2XeF2 H2XeF4 XeO3 H2XeOarrow_forwardWhile noble gas exerts the strongest London (dispersion) forces on neighboring atoms? Group of answer choices Xe Ar Kr Nearrow_forward
- Which of the following elements is corrosive to your skin due to that element breaking down C=C bonds? Group of answer choices fluorine iodine bromine chlorinearrow_forwardWhat the best source of sulfide to use on a small scale in the lab? Group of answer choices thiourea H2S NaHS Na2Sarrow_forwardWhich of the following statements about sulfur is FALSE? Group of answer choices H2S is the product of an oxygen-depleted ecosystem. In the acid mine drainage reaction, FeS2 is a product. One allotrope of sulfur has the formula S20. In the environment, bacterial oxidation can convert S2− to elemental S or SO42−.arrow_forward
- Of the following choices, which is the best reason that most materials DON'T spontaneously combust even though our atmosphere is about 21% oxygen? Group of answer choices The reduction of O2 in the gas phase (O2 + e− → O2−) is spontaneous. The reduction of O2 in acid solution (O2 + H+ + e− → HO2(aq)) is spontaneous. O2 is not a reactant in combustion. The O2 bond dissociation energy is 494 kJ/mol, leading to a high activation energy for combustion.arrow_forwardplease answer in the scope of the SCH4U course, I am having a hard time understanding, may you show all steps please and thank you! can you also put the final answers in the table so its understandablearrow_forwardPlan the synthesis of the following compound using the starting material provided and any other reagents needed as long as carbon based reagents have 3 carbons or less. Either the retrosynthesis or the forward synthesis (mechanisms are not required but will be graded if provided) will be accepted if all necessary reagents and intermediates are shown (solvents and temperature requirements are not needed unless specifically involved in the reaction, i.e. DMSO in the Swem oxidation or heat in the KMnO4 oxidation). There may be more than one correct answer, and chemically correct steps will be accepted. Extra points will be given if correct names are provided. The points earned here will be applied to your lowest exam score! H Harrow_forward
- Draw the mechanism to make the alcohol 1-hexanol. Please use arrows.arrow_forwardAnswer the followings: 1-What is the difference(s) between DNA and RNA: a- Structure: b- Function: c- Types: 2-What is the meaning of: a- Replication b- Transcription c- Translation 3- Show the base pair connection (hydrogen bond) in DNA and RNAarrow_forwardWhy does the anhydride react with the OH on the benzene rather than the OH on the carboxy group?arrow_forward
