
Interpretation:
The product of the reaction for given two reactants should be drawn.
Concept introduction:
In a sigmatropic reaction “ one new sigma-bond is formed as another breaks.”
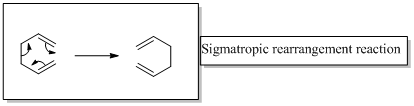
Sigma tropic rearrangement reactions are designated with digits. For example a [1, 3] sigma tropic rearrangement describe a reaction in which the residue migrates from position 1 to position 3.
Woodward –Hoffmann rules are the set of rules used to vindicate or predict certain aspects of the stereo chemical outcome and activation energy of pericyclic reactions.
Woodward – Hoffmann rules for sigma tropic rearrangement reactions are listed below
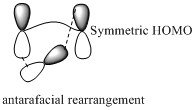
Migration of carbon and hydrogen will occur in a sigmatropic rearrangement reaction. Migration of hydrogen in suprafacial and antarafacial rearrangement can be represented as follows,


Carbon migrating with one lobe of its p orbital interacting
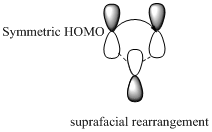

Carbon migrating with both lobe of its p orbital interacting

Trending nowThis is a popular solution!

Chapter 28 Solutions
Organic Chemistry
- V Biological Macromolecules Drawing the Haworth projection of an aldose from its Fischer projection Draw a Haworth projection of a common cyclic form of this monosaccharide: H C=O HO H HO H H OH CH₂OH Explanation Check Click and drag to start drawing a structure. Xarrow_forwardComplete the mechanismarrow_forwardComplete the mechanismarrow_forward
- 8 00 6 = 10 10 Decide whether each of the molecules in the table below is stable, in the exact form in which it is drawn, at pH = 11. If you decide at least one molecule is not stable, then redraw one of the unstable molecules in its stable form below the table. (If more than unstable, you can pick any of them to redraw.) Check OH stable HO stable Ounstable unstable O OH stable unstable OH 80 F6 F5 stable Ounstable X Save For Later Sub 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C ཀྭ་ A F7 매 F8 F9 4 F10arrow_forwardJust try completing it and it should be straightforward according to the professor and TAs.arrow_forwardThe grading is not on correctness, so if you can just get to the correct answers without perfectionism that would be great. They care about the steps and reasoning and that you did something. I asked for an extension, but was denied the extension.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
