
Concept explainers
Show the products you would expect to obtain from reaction of glyceryl Trioleate with the following reagents:
(a) Excess Br2 in CH2Cl2
(b) H2/Pd
(c) NaOH/H2O
(d) O3, then Zn/CH3CO2H
(e) LiAlH4, then H3O1
(f) CH3MgBr, then H3O1
a) Excess Br2 in CH2Cl2
Interpretation:
The product expected to be formed when glyceryl trioleate reacts with excess bromine in CH2Cl2 is to be given.
Concept introduction:
When compounds containing more than one olefinic double bond are treated with bromine in excess, addition of bromine to all the olefinic double bonds in the molecule takes place.
To give:
The product expected to be formed when glyceryl trioleate reacts with excess bromine in CH2Cl2.
Answer to Problem 20AP
The product expected to be formed when glyceryl trioleate reacts with excess bromine in CH2Cl2 is
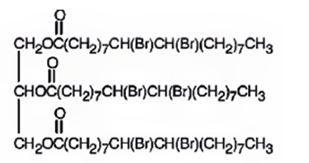
Explanation of Solution
Glyceryl trioleate has three olefinic diuble bonds. When glyceryl trioleate is treated with excess bromine in CH2Cl2 addition of bromine takes place to all the three double bonds in the molecule.
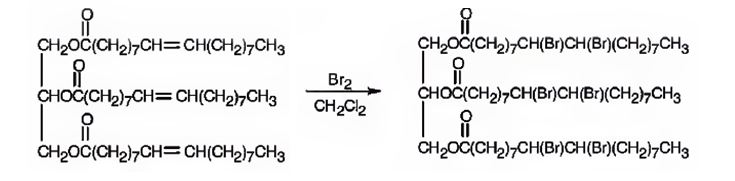
The product expected to be formed when glyceryl trioleate reacts with excess bromine in CH2Cl2 is

b) H2/Pd
Interpretation:
The product expected to be formed when glyceryl trioleate reacts with H2/Pd is to be given.
Concept introduction:
When compounds containing more than one olefinic double bond are treated with H2/Pd, reduction occurs and addition of hydrogen to all the double bonds in the molecule takes place.
To give:
The product expected to be formed when glyceryl trioleate reacts with H2/Pd.
Answer to Problem 20AP
The product expected to be formed when glyceryl trioleate reacts with H2/Pd is
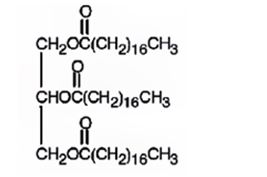
Explanation of Solution
When glyceryl trioleate is treated with H2/Pd, addition of hydrogen takes place to the three double bonds in the molecule to yield a saturated compound.
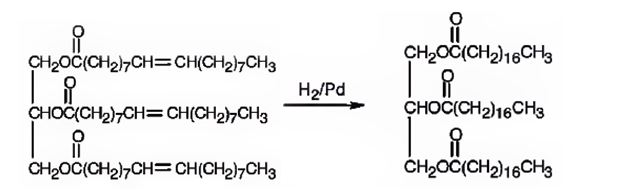
The product expected to be formed when glyceryl trioleate reacts with H2/Pd is

c) NaOH/H2O
Interpretation:
The products expected to be formed when glyceryl trioleate reacts with NaOH/H2O are to be given.
Concept introduction:
When an oil or fat is treated with aqueous NaOH, the ester linkages in them get hydrolyzed to yield glycerol and sodium salt of the acid.
To give:
The products expected to be formed when glyceryl trioleate reacts with NaOH/H2O.
Answer to Problem 20AP
The products expected to be formed when glyceryl trioleate reacts with NaOH/H2O are given below.
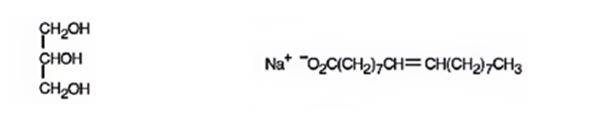
Explanation of Solution
When glyceryl trioleate is treated with aqueous NaOH, the ester linkages in them get hydrolyzed to yield glycerol and sodium oleate.
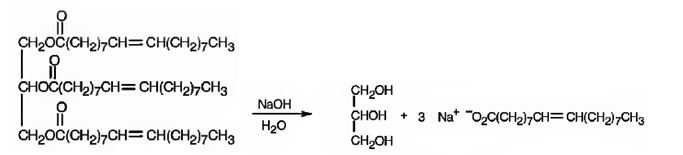
The products expected to be formed when glyceryl trioleate reacts with NaOH/H2O are given below.

d) O3, then Zn/CH3CO2H
Interpretation:
The products expected to be formed when glyceryl trioleate reacts with O3, then with Zn/CH3COOH are to be given.
Concept introduction:
When a compound with double bond is treated with ozone and then with Zn/ CH3COOH, the double bond gets cleaved and the products have an oxygen atom attached to each carbon originally in the double bond.
To give:
The products expected to be formed when glyceryl trioleate reacts with O3, then with Zn/CH3COOH.
Answer to Problem 20AP
The products expected to be formed when glyceryl trioleate reacts with O3, then with Zn/CH3COOH are given below.

Explanation of Solution
When glyceryl trioleate is treated with O3 and then with Zn/CH3COOH, ozone adds to the three double bonds to yield a triozonide which is cleaved when treated with Zn/CH3COOH to give two different aldehydes as products. The aldehyde carbons are present in a double bond in glyceryl trioleate.
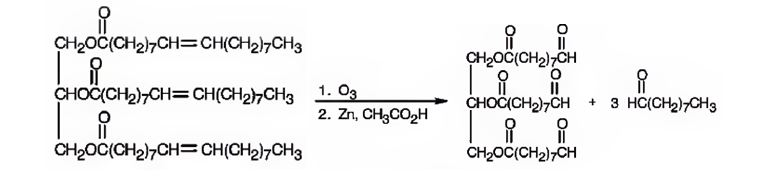
The products expected to be formed when glyceryl trioleate reacts with O3, then with Zn/CH3COOH are given below.

e) LiAlH4, then H3O+
Interpretation:
The products expected to be formed when glyceryl trioleate reacts with LiAlH4, then with H3O+ are to be given.
Concept introduction:
When oils and fats are reduced with LiAlH4, then with H3O+, the eater linkages are reduced to give glycerol and the free acid. Double bonds are not reduced by LiAlH4.
To give:
The products expected to be formed when glyceryl trioleate reacts with LiAlH4, then with H3O+.
Answer to Problem 20AP
The products expected to be formed when glyceryl trioleate reacts with LiAlH4, then with H3O+ are given below.
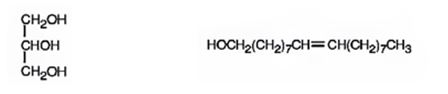
Explanation of Solution
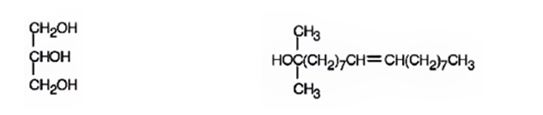
When glyceryl trioleate is treated with LiAlH4 and then with H3O+ the ester linkages in it are reduced to alcohols. LiAlH4 does not reduce the double bonds in glyceryl trioleate.
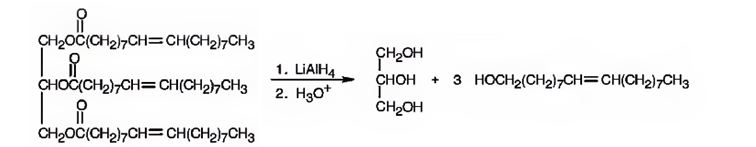
The products expected to be formed when glyceryl trioleate reacts with LiAlH4, then with H3O+ are given below.

f) CH3MgBr, then H3O+
Interpretation:
The products expected to be formed when glyceryl trioleate reacts with CH3MgBr, then with H3O+ are to be given.
Concept introduction:
Esters when treated with a Grignard reagent and then with H3O+, react with two equivalents of the Grignard reagent to yield a tertiary alcohol. In oils and fats addition of Grignard reagent will take place to all the three carbonyl groups in the ester.
To give:
The products expected to be formed when glyceryl trioleate reacts with CH3MgBr, then with H3O+.
Answer to Problem 20AP
The products expected to be formed when glyceryl trioleate reacts with CH3MgBr, then with H3O+ are given below.
Explanation of Solution
When glyceryl trioleate is treated with CH3MgBr, addition to carbonyl carbons takes place. The addition product when hydrolyzed with H3O+ yields three equivalents of a tertiary alcohol and glycerol as products.
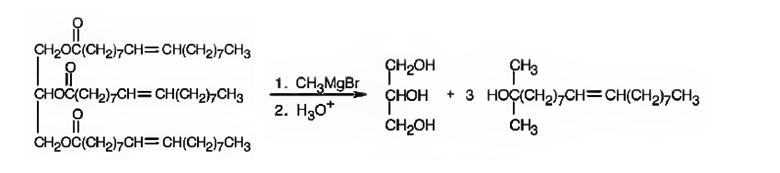
The products expected to be formed when glyceryl trioleate reacts with CH3MgBr, then with H3O+ are given below.

Want to see more full solutions like this?
Chapter 27 Solutions
Student Value Bundle: Organic Chemistry, + OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card (NEW!!)
Additional Science Textbook Solutions
Genetics: From Genes to Genomes
Physical Universe
Campbell Biology (11th Edition)
General, Organic, and Biological Chemistry - 4th edition
- 9. Write Me product as well as the reaction Mechanism For each of the Following Vanctions +H₂504 4.50+ T C. +212 Fellz 237 b. Praw the potential energy Diagrams For each OF Mese Rauctions and account For any differences that appear in the two potential Puergy Diagrams which of here two reactions 19 Found to be Reversable, Rationalice your answer based upon the venation mechanisms and the potential energy diagrams.arrow_forward9. Write Me product as well as the reaction Mechanism For each of the Following Veritious +H2504 4.50+ + 1/₂ Felly ◎+ 7 b. Praw he potential energy Diagrams For each OF Mese Ronctions and account for any differences that appeak in the two potential Puergy Diagramsarrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Incorrect, 3 attempts remaining 1. excess Br2, NaOH 2. neutralizing workup Qarrow_forward
- Given the electrode Pt | Ag | Ag+ (aq), describe it.arrow_forwardAt 25°C, the reaction Zn2+ + 2e ⇄ Zn has a normal equilibrium potential versus the saturated calomel electrode of -1.0048 V. Determine the normal equilibrium potential of Zn versus the hydrogen electrode.Data: The calomel electrode potential is E° = 0.2420 V versus the normal hydrogen electrode.arrow_forwardElectrochemistry. State the difference between E and E0.arrow_forward
- In an electrolytic cell, the positive pole is always assumed to be on the right side of the battery notation. Is that correct?arrow_forwardIn an electrolytic cell, the positive pole is always assumed to be on the right side of the battery. Is that correct?arrow_forwardCalculate the free energy of formation of 1 mol of Cu in cells where the electrolyte is 1 mol dm-3 Cu2+ in sulfate solution, pH 0. E° for the Cu2+/Cu pair in this medium is +142 mV versus ENH.Assume the anodic reaction is oxygen evolution.Data: EH2 = -0.059 pH (V) and EO2 = 1.230 - 0.059 pH (V); 2.3RT/F = 0.059 Varrow_forward
- If the normal potential for the Fe(III)/Fe(II) pair in acid at zero pH is 524 mV Hg/Hg2Cl2 . The potential of the saturated calomel reference electrode is +246 mV versus the NHE. Calculate E0 vs NHE.arrow_forwardGiven the galvanic cell whose scheme is: (-) Zn/Zn2+ ⋮⋮ Ag+/Ag (+). If we know the normal potentials E°(Zn2+/Zn) = -0.76V and E°(Ag+/Ag) = 0.799 V. Indicate the electrodes that are the anode and the cathode and calculate the E0battery.arrow_forwardIndicate the functions that salt bridges have in batteries.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

