
Concept explainers
Show the products you would expect to obtain from reaction of glyceryl Trioleate with the following reagents:
(a) Excess Br2 in CH2Cl2
(b) H2/Pd
(c) NaOH/H2O
(d) O3, then Zn/CH3CO2H
(e) LiAlH4, then H3O1
(f) CH3MgBr, then H3O1
a) Excess Br2 in CH2Cl2
Interpretation:
The product expected to be formed when glyceryl trioleate reacts with excess bromine in CH2Cl2 is to be given.
Concept introduction:
When compounds containing more than one olefinic double bond are treated with bromine in excess, addition of bromine to all the olefinic double bonds in the molecule takes place.
To give:
The product expected to be formed when glyceryl trioleate reacts with excess bromine in CH2Cl2.
Answer to Problem 20AP
The product expected to be formed when glyceryl trioleate reacts with excess bromine in CH2Cl2 is
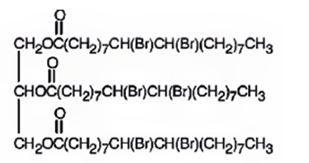
Explanation of Solution
Glyceryl trioleate has three olefinic diuble bonds. When glyceryl trioleate is treated with excess bromine in CH2Cl2 addition of bromine takes place to all the three double bonds in the molecule.
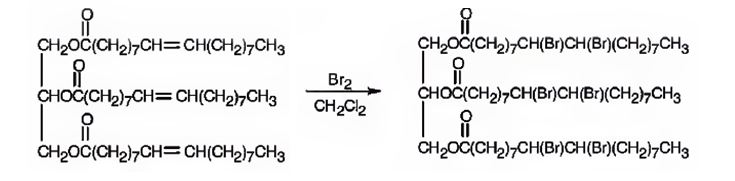
The product expected to be formed when glyceryl trioleate reacts with excess bromine in CH2Cl2 is

b) H2/Pd
Interpretation:
The product expected to be formed when glyceryl trioleate reacts with H2/Pd is to be given.
Concept introduction:
When compounds containing more than one olefinic double bond are treated with H2/Pd, reduction occurs and addition of hydrogen to all the double bonds in the molecule takes place.
To give:
The product expected to be formed when glyceryl trioleate reacts with H2/Pd.
Answer to Problem 20AP
The product expected to be formed when glyceryl trioleate reacts with H2/Pd is
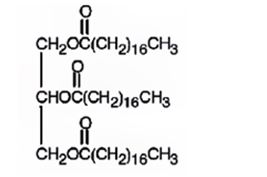
Explanation of Solution
When glyceryl trioleate is treated with H2/Pd, addition of hydrogen takes place to the three double bonds in the molecule to yield a saturated compound.
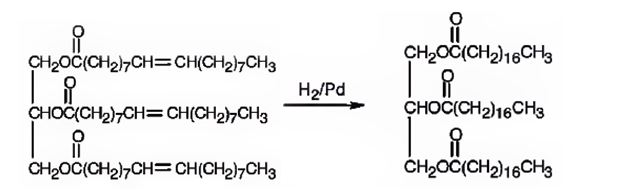
The product expected to be formed when glyceryl trioleate reacts with H2/Pd is

c) NaOH/H2O
Interpretation:
The products expected to be formed when glyceryl trioleate reacts with NaOH/H2O are to be given.
Concept introduction:
When an oil or fat is treated with aqueous NaOH, the ester linkages in them get hydrolyzed to yield glycerol and sodium salt of the acid.
To give:
The products expected to be formed when glyceryl trioleate reacts with NaOH/H2O.
Answer to Problem 20AP
The products expected to be formed when glyceryl trioleate reacts with NaOH/H2O are given below.
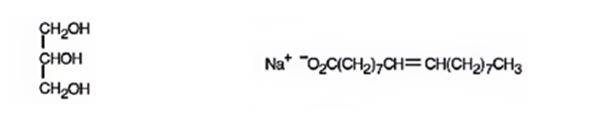
Explanation of Solution
When glyceryl trioleate is treated with aqueous NaOH, the ester linkages in them get hydrolyzed to yield glycerol and sodium oleate.
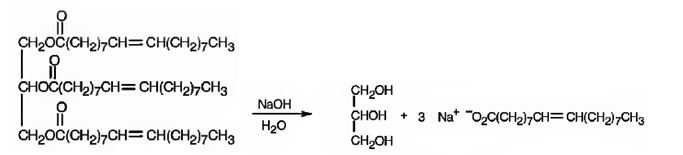
The products expected to be formed when glyceryl trioleate reacts with NaOH/H2O are given below.

d) O3, then Zn/CH3CO2H
Interpretation:
The products expected to be formed when glyceryl trioleate reacts with O3, then with Zn/CH3COOH are to be given.
Concept introduction:
When a compound with double bond is treated with ozone and then with Zn/ CH3COOH, the double bond gets cleaved and the products have an oxygen atom attached to each carbon originally in the double bond.
To give:
The products expected to be formed when glyceryl trioleate reacts with O3, then with Zn/CH3COOH.
Answer to Problem 20AP
The products expected to be formed when glyceryl trioleate reacts with O3, then with Zn/CH3COOH are given below.

Explanation of Solution
When glyceryl trioleate is treated with O3 and then with Zn/CH3COOH, ozone adds to the three double bonds to yield a triozonide which is cleaved when treated with Zn/CH3COOH to give two different aldehydes as products. The aldehyde carbons are present in a double bond in glyceryl trioleate.
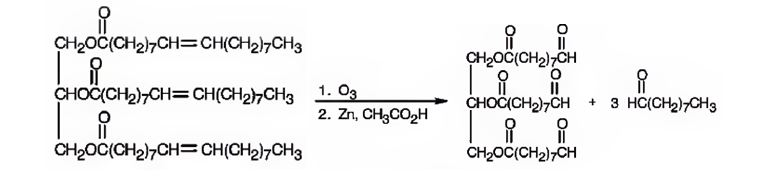
The products expected to be formed when glyceryl trioleate reacts with O3, then with Zn/CH3COOH are given below.

e) LiAlH4, then H3O+
Interpretation:
The products expected to be formed when glyceryl trioleate reacts with LiAlH4, then with H3O+ are to be given.
Concept introduction:
When oils and fats are reduced with LiAlH4, then with H3O+, the eater linkages are reduced to give glycerol and the free acid. Double bonds are not reduced by LiAlH4.
To give:
The products expected to be formed when glyceryl trioleate reacts with LiAlH4, then with H3O+.
Answer to Problem 20AP
The products expected to be formed when glyceryl trioleate reacts with LiAlH4, then with H3O+ are given below.
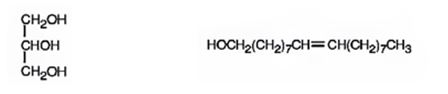
Explanation of Solution
When glyceryl trioleate is treated with LiAlH4 and then with H3O+ the ester linkages in it are reduced to alcohols. LiAlH4 does not reduce the double bonds in glyceryl trioleate.
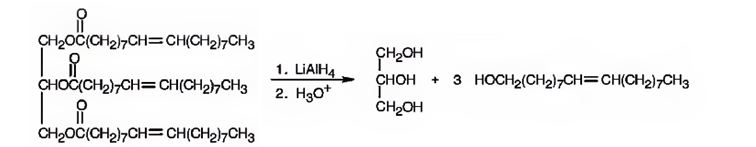
The products expected to be formed when glyceryl trioleate reacts with LiAlH4, then with H3O+ are given below.

f) CH3MgBr, then H3O+
Interpretation:
The products expected to be formed when glyceryl trioleate reacts with CH3MgBr, then with H3O+ are to be given.
Concept introduction:
Esters when treated with a Grignard reagent and then with H3O+, react with two equivalents of the Grignard reagent to yield a tertiary alcohol. In oils and fats addition of Grignard reagent will take place to all the three carbonyl groups in the ester.
To give:
The products expected to be formed when glyceryl trioleate reacts with CH3MgBr, then with H3O+.
Answer to Problem 20AP
The products expected to be formed when glyceryl trioleate reacts with CH3MgBr, then with H3O+ are given below.
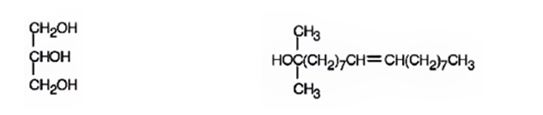
Explanation of Solution
When glyceryl trioleate is treated with CH3MgBr, addition to carbonyl carbons takes place. The addition product when hydrolyzed with H3O+ yields three equivalents of a tertiary alcohol and glycerol as products.
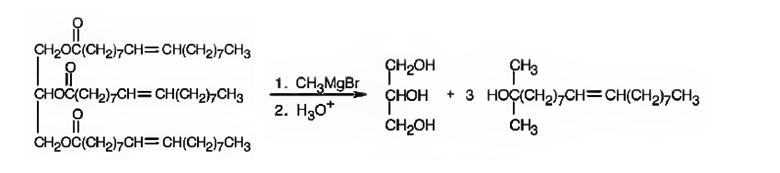
The products expected to be formed when glyceryl trioleate reacts with CH3MgBr, then with H3O+ are given below.

Want to see more full solutions like this?
Chapter 27 Solutions
EBK ORGANIC CHEMISTRY
Additional Science Textbook Solutions
Genetics: From Genes to Genomes
Physical Universe
Campbell Biology (11th Edition)
General, Organic, and Biological Chemistry - 4th edition
- Step 1: add a curved arrow. Select Draw Templates More / " C H Br 0 Br : :o: Erase H H H H Q2Q Step 2: Draw the intermediates and a curved arrow. Select Draw Templates More MacBook Air / " C H Br 0 9 Q Erase 2Qarrow_forwardO Macmillan Learning Question 23 of 26 > Stacked Step 7: Check your work. Does your synthesis strategy give a substitution reaction with the expected regiochemistry and stereochemistry? Draw the expected product of the forward reaction. - - CN DMF MacBook Air Clearly show stereochemistry. Questionarrow_forwardNH2 1. CH3–MgCl 2. H3O+ ? As the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will make a molecule with a new C - C bond as its major product: If this reaction will work, draw the major organic product or products you would expect in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If the major products of this reaction won't have a new C - C bond, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. This reaction will not make a product with a new C - C bond. Х ☐: Carrow_forward
- Predict the major products of this organic reaction. If there will be no major products, check the box under the drawing area instead. No reaction. : + Х è OH K Cr O 2 27 2 4' 2 Click and drag to start drawing a structure.arrow_forwardLaminar compounds are characterized by havinga) a high value of the internal surface of the solid.b) a high adsorption potential.arrow_forwardIntercalation compounds have their sheetsa) negatively charged.b) positively charged.arrow_forward
- Indicate whether the following two statements are correct or not:- Polythiazine, formed by N and S, does not conduct electricity- Carbon can have a specific surface area of 3000 m2/garrow_forwardIndicate whether the following two statements are correct or not:- The S8 heterocycle is the origin of a family of compounds- Most of the elements that give rise to stable heterocycles belong to group d.arrow_forwardcould someone draw curly arrow mechanism for this question pleasearrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

