
Tutorials In Introductory Physics: Homework
1st Edition
ISBN: 9780130662453
Author: Lillian C. McDermott, Peter S. Shaffer
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 27.2, Problem 2dTH
One mole of an ideal gas is confined to a container with a movable piston. The questions below refer to the processes shown on the PV diagram at right. Process I is a change front state X to state Y at constant pressure. Process II is a change from state W to state Z at a different constant pressure.
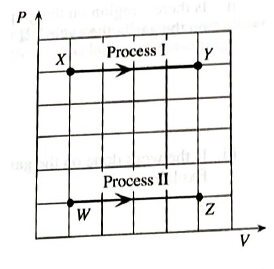
d. In Process I, is the
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
In the movie Fast X, a 10100 kg round bomb is set rolling in Rome. The bomb gets up to 17.6 m/s. To try to stop the bomb, the protagonist Dom swings the counterweight of a crane, which has a mass of 354000 kg into the bomb at 3.61 m/s in the opposite direction. Directly after the collision the crane counterweight continues in the same direction it was going at 2.13 m/s. What is the velocity (magnitude and direction) of the bomb right after the collision?
Don't use ai
Make sure to draw a sketch with scale please
Chapter 27 Solutions
Tutorials In Introductory Physics: Homework
Ch. 27.1 - Prob. 1aTHCh. 27.1 - In this process, which of the quantities P, V, n,...Ch. 27.1 - Consider the following incorrect student...Ch. 27.1 - Explain why it is not possible to use the ideal...Ch. 27.1 - A long pin is used to hold the piston in place as...Ch. 27.1 - A long pin is used to hold the piston in place as...Ch. 27.1 - Prob. 2cTHCh. 27.2 - Prob. 1aTHCh. 27.2 - Prob. 1bTHCh. 27.2 - Prob. 1cTH
Additional Science Textbook Solutions
Find more solutions based on key concepts
14. An experienced goldfish breeder receives two unusual male goldfish. One is black rather than gold, and the ...
Genetic Analysis: An Integrated Approach (3rd Edition)
The enzyme that catalyzes the C C bond cleavage reaction that converts serine to glycine removes the substitue...
Organic Chemistry (8th Edition)
45. A 140 g baseball is moving horizontally to the right at35 m/s when it is hit by the bat. The ball flies off...
College Physics: A Strategic Approach (3rd Edition)
1. ___ Mitosis 2. ___ Meiosis 3. __ Homologous chromosomes 4. __ Crossing over 5. __ Cytokinesis A. Cytoplasmic...
Microbiology with Diseases by Body System (5th Edition)
Plants use the process of photosynthesis to convert the energy in sunlight to chemical energy in the form of su...
Campbell Essential Biology (7th Edition)
The following data were obtained from a disk-diffusion test. Antibiotic Zone of Inhibition A 15 mm B 0 mm c 7 m...
Microbiology: An Introduction
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Make sure to draw a sketch with scalearrow_forwardUltimate Byleth and Little Mac fight. Little Mac, who is a boxer, dashes forward at 26.6 m/s, fist first. Byleth moves in the opposite direction at 3.79 m/s, where they collide with Little Mac’s fist. After the punch Byleth flies backwards at 11.1 m/s. How fast, and in what direction, is Little Mac now moving? Little Mac has a mass of 48.5 kg and Byleth has a mass of 72.0 kg.arrow_forwardMake sure to draw a sketch with scale as wellarrow_forward
- Make sure to draw a sketch with scale pleasearrow_forwardKirby jumps towards his enemy/ally, Meta Knight, at 2.06 m/s while Meta Knight glides in the opposite direction (toward Kirby) at 5.06 m/s. Kirby then begins to inhale, swallowing Meta Knight. What is Kirby/Meta Knight’s velocity immediately after being swallowed? Please put the magnitude of the velocity and then mark direction using dropdown menu. Kirby has a mass of 0.283 kg and Meta Knight has a mass of 0.538 kg.arrow_forwardNo Aiarrow_forward
- Can someone help mearrow_forwardNeed help on the following questions on biomechanics. (Please refer to images below)A gymnast weighing 68 kg attempts a handstand using only one arm. He plants his handat an angle resulting in the reaction force shown.A) Find the resultant force (acting on the Center of Mass)B) Find the resultant moment (acting on the Center of Mass)C) Draw the resultant force and moment about the center of mass on the figure below. Will the gymnast rotate, translate, or both? And in which direction?arrow_forwardPlease help me on the following question (Please refer to image below)An Olympic lifter (m = 103kg) is holding a lift with a mass of 350 kg. The barexerts a purely vertical force that is equally distributed between both hands. Each arm has amass of 9 kg, are 0.8m long and form a 40° angle with the horizontal. The CoM for each armis 0.5 m from hand. Assuming the lifter is facing us in the diagram below, his right deltoidinserts 14cm from the shoulder at an angle of 13° counter-clockwise from the humerus.A) You are interested in calculating the force in the right deltoid. Draw a free body diagramof the right arm including the external forces, joint reaction forces, a coordinate system andstate your assumptions.B) Find the force exerted by the right deltoidC) Find the shoulder joint contact force. Report your answer using the magnitude and directionof the shoulder force vector.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY