
Concept explainers
Oligonucleotide Synthesis
In Section
available for use as primers for PCR and as probes for cloning DNA. Here we will examine how these oligonucleotides are prepared.
The method bears many similarities to the Merrifield solid-phase synthesis of peptides. A starter unit is attached to a solid support, nucleosides are attached one-by-one until the sequence is complete, whereupon the target oligonucleotide is removed from the support and purified. Like solid-phase peptide synthesis, the preparation of oligonucleotides relies heavily on protecting groups and bond-forming methods.
The starter units are nucleosides in which
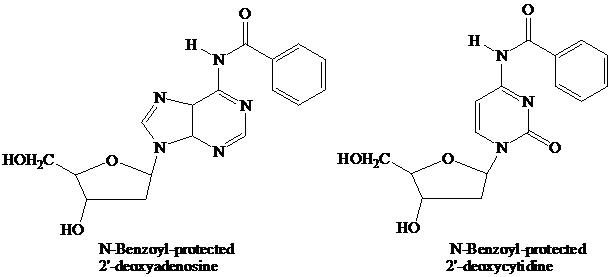
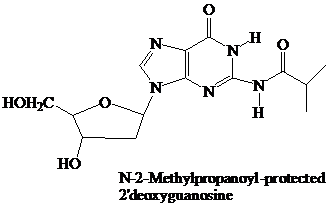
Thymidine lacks an
These
The
(DMT) ether.
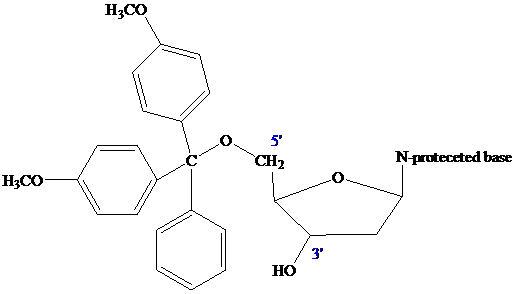
The nucleoside that is to serve as the
controlled-pore glass (CPG) bead by ester formation between its unprotected

The stage is now set for adding the second nucleoside. The four blocked nucleosides prepared
earlier are converted to their corresponding
derivatives. An appropriate A, C, T, or G phosphoramidite is used in each successive stage of the elongation cycle.
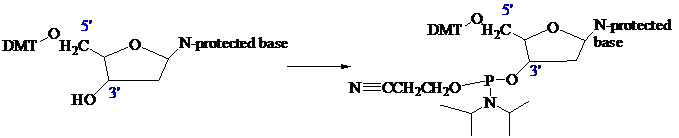
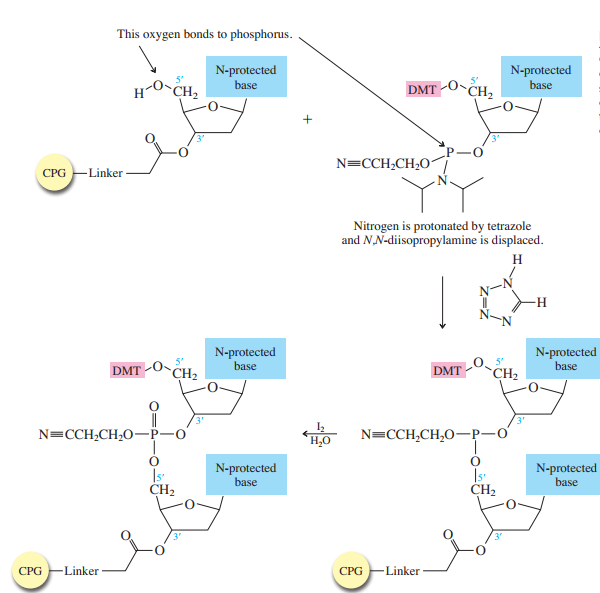
Each phosphoramidite is coupled to the anchored nucleoside by a reaction in which the free
The product of the coupling is a phosphite; it has the general formula
in the last step of Figure
The

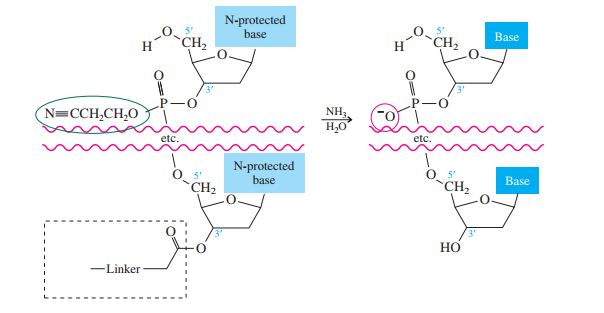
Once all the nucleosides are in place and the last DMT is removed, treatment with aqueous
ammonia removes the acyl and cyanoethyl groups and cleaves the oligonucleotide from the CPG
support.
Structure 1 is the one given for tetrazole in Figure
same molecular formula
structures related?
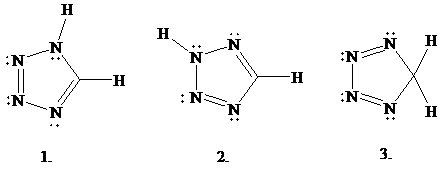
A.
B.
C.
D.
Section 27.6 Many important compounds contain two or more nucleotides joined together by
a phosphodiester linkage. The best known are those in which the phosphodiester joins the
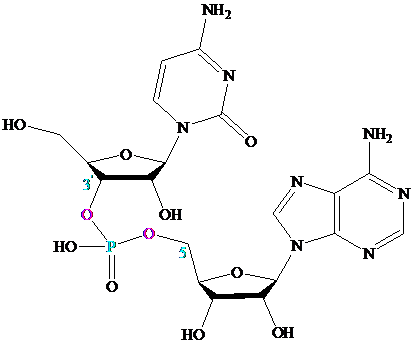
Oligonucleotides contain about
phosphodiester links; polynucleotides can contain thousands of nucleotides.
Want to see the full answer?
Check out a sample textbook solution
Chapter 27 Solutions
ORGANIC CHEMISTRY (LOOSELEAF)-PACKAGE
- if the answer is no reaction than state that and please hand draw!arrow_forward"I have written solutions in text form, but I need experts to rewrite them in handwriting from A to Z, exactly as I have written, without any changes."arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- Please correct answer and don't used hand raitingarrow_forwardreciprocal lattices rotates along with the real space lattices of the crystal. true or false?arrow_forwardDeducing the reactants of a Diels-Alder reaction vn the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? Δ O If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. Click and drag to start drawing a structure. Product can't be made in one step. Explanation Checkarrow_forward
- Predict the major products of the following organic reaction: Δ ? Some important notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Explanation Check Click and drag to start drawing a structure. Larrow_forward> Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? Δ • If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. If your answer is no, check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accesarrow_forwardPredict the major products of the following organic reaction: O O + A ? Some important notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Explanation Check Click and drag to start drawing a structure. eserved. Terms of Use | Privacy Center >arrow_forward

 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





