
Organic Chemistry
4th Edition
ISBN: 9780073402772
Author: Janice G. Smith
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 27, Problem 27.11P
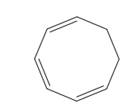
What product would be formed by the disrotatory cyclization of the given triene? Would this reaction occur under photochemical or thermal conditions?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
In one electrode: Pt, H2(1 atm) | H+(a=1), the interchange current density at 298K is 0.79 mA·cm-2. If the voltage difference of the interface is +5 mV. What will be the correct intensity at pH = 2?. Maximum transfer voltage and beta = 0.5.
In a Pt electrode, H2(1 atm) | H+(a=1), the interchange current density of an electrode is 0.79 mA cm-2. ¿Qué corriente flow across the electrode of área 5 cm2 when the difference in potential of the interface is +5 mV?.
If the current voltage is n = 0.14 V, indicate which of the 2 voltage
formulas of the ley of Tafel must be applied
i
a
a) == exp (1-B).
xp[(1 - ß³):
Fn
Fn
a
b) == exp B
RT
RT
Chapter 27 Solutions
Organic Chemistry
Ch. 27 - Prob. 27.1PCh. 27 - Problem 27.2
For each molecular orbital in Figure...Ch. 27 - Problem 27.3
(a) Using Figure 27.2 as a guide,...Ch. 27 - Problem 27.4
(a) How many molecular orbitals are...Ch. 27 - Prob. 27.5PCh. 27 - Prob. 27.6PCh. 27 - Prob. 27.7PCh. 27 - Prob. 27.8PCh. 27 - Prob. 27.9PCh. 27 - Prob. 27.10P
Ch. 27 - Problem 27.11
What product would be formed by the...Ch. 27 - Consider cycloheptatrienone and ethylene, and draw...Ch. 27 - Problem 27.13
Show that a thermal suprafacial...Ch. 27 - Prob. 27.14PCh. 27 - a Draw the product of the following [4+2]...Ch. 27 - Prob. 27.16PCh. 27 - Prob. 27.17PCh. 27 - Problem 27.18
Using orbital symmetry, explain why...Ch. 27 - Prob. 27.19PCh. 27 - Prob. 27.20PCh. 27 - Prob. 27.21PCh. 27 - Prob. 27.22PCh. 27 - Prob. 27.23PCh. 27 - Problem 27.25
(a) What product is formed by the...Ch. 27 - Prob. 27.25PCh. 27 - Prob. 27.26PCh. 27 - Prob. 27.27PCh. 27 - Prob. 27.28PCh. 27 - Prob. 27.29PCh. 27 - Prob. 27.30PCh. 27 - Prob. 27.31PCh. 27 - Prob. 27.32PCh. 27 - Prob. 27.33PCh. 27 - Prob. 27.34PCh. 27 - Prob. 27.35PCh. 27 - Prob. 27.36PCh. 27 - Prob. 27.37PCh. 27 - Prob. 27.38PCh. 27 - Prob. 27.39PCh. 27 - Prob. 27.40PCh. 27 - Prob. 27.41PCh. 27 - Prob. 27.42PCh. 27 - Prob. 27.43PCh. 27 - Prob. 27.44PCh. 27 - Prob. 27.45PCh. 27 - 27.47 What product is formed from the [5,5]...Ch. 27 - 27.49 Draw structures for A, B, and C in the...Ch. 27 - Prob. 27.48PCh. 27 - Prob. 27.49PCh. 27 - 27.52 Draw the products of each reaction.
c....Ch. 27 - Prob. 27.51PCh. 27 - 27.54 Draw a stepwise, detailed mechanism for the...Ch. 27 - Prob. 27.53PCh. 27 - Prob. 27.54PCh. 27 - 27.58 Draw a stepwise, detailed mechanism for the...Ch. 27 - Prob. 27.56PCh. 27 - Prob. 27.57PCh. 27 - Prob. 27.58PCh. 27 - Prob. 27.59P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If the current voltage is n = 0.14 V. Indicate which of the 2 formulas must be applied a) = a T = i exp[(1 - p) F Fn Fn b) i==exp B RTarrow_forwardTopic: Photochemistry and Photophysics of Supramoleculesarrow_forwardTwo cations that exchange an electron in an interface, the exchange density is worth 1.39 mA/cm2 and the current density is worth 15 mA/cm2 at 25°C. If the overvoltage is 0.14 V, calculate the reaction rate and symmetry factor. Data: R = 8,314 J mol-1 k-1: F = 96500 Carrow_forward
- With the help of the Tafel line, it is estimated that the interchange density of the VO2+/VO2+ system on the carbon paper has a value of 3 mA cm-2. Calculate a) the current density if the voltage has a value of 1.6 mV and the temperature is 25°C. b) the beta value of the anódico process if the Tafel pendulum is 0.6 V at 25°C. Data: R = 8.314 JK-1mol-1, y F = 96485 C mol-1.arrow_forwardApply the NANSTE law to the MnO4- + 8H+ + 5e- ⇄ Mn2+ + 4H2Oarrow_forwardIn the Nernst Law, how much is RT / F?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY