
Concept explainers
The phosphorylation of
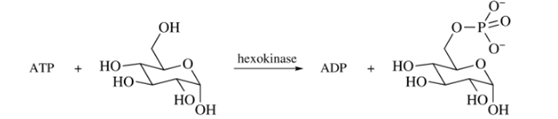
Is this reaction exergonic or endergonic?
How would the value of
Use the value for the hydrolysis of ATP to ADP (Section 27.5) to calculate
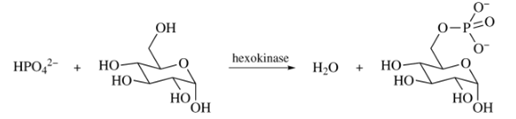
Want to see the full answer?
Check out a sample textbook solution
Chapter 27 Solutions
ORGANIC CHEMISTRY (LL)-W/SOLN.>CUSTOM<
- Q2: Draw the molecules based on the provided nomenclatures below: (2R,3S)-2-chloro-3-methylpentane: (2S, 2R)-2-hydroxyl-3,6-dimethylheptane:arrow_forwardQ3: Describes the relationship (identical, constitutional isomers, enantiomers or diastereomers) of each pair of compounds below. ག H CH3 OH OH CH3 H3C OH OH OH ////////// C CH3 CH3 CH3 CH3 H3C CH 3 C/III..... Physics & Astronomy www.physics.northweste COOH H нош..... H 2 OH HO CH3 HOOC H CH3 CH3 CH3 Br. H H Br and H H H Harrow_forwardQ1: For each molecule, assign each stereocenter as R or S. Circle the meso compounds. Label each compound as chiral or achiral. OH HO CI Br H CI CI Br CI CI Xf x f g Br D OH Br Br H₂N R. IN Ill I -N S OMe D II H CO₂H 1/111 DuckDuckGarrow_forward
- These are synthesis questions. You need to show how the starting material can be converted into the product(s) shown. You may use any reactions we have learned. Show all the reagents you need. Show each molecule synthesized along the way and be sure to pay attention to the regiochemistry and stereochemistry preferences for each reaction. If a racemic molecule is made along the way, you need to draw both enantiomers and label the mixture as "racemic". All of the carbon atoms of the products must come from the starting material! ? H Harrow_forwardQ5: Draw every stereoisomer for 1-bromo-2-chloro-1,2-difluorocyclopentane. Clearly show stereochemistry by drawing the wedge-and-dashed bonds. Describe the relationship between each pair of the stereoisomers you have drawn.arrow_forwardClassify each pair of molecules according to whether or not they can participate in hydrogen bonding with one another. Participate in hydrogen bonding CH3COCH3 and CH3COCH2CH3 H2O and (CH3CH2)2CO CH3COCH3 and CH₂ CHO Answer Bank Do not participate in hydrogen bonding CH3CH2OH and HCHO CH3COCH2CH3 and CH3OHarrow_forward
- Nonearrow_forwardGiven the standard enthalpies of formation for the following substances, determine the reaction enthalpy for the following reaction. 4A (g) + 2B (g) → 2C (g) + 7D (g) AHrxn =?kJ Substance AH in kJ/mol A (g) - 20.42 B (g) + 32.18 C (g) - 72.51 D (g) - 17.87arrow_forwardDetermine ASran for Zn(s) + 2HCl(aq) = ZnCl2(aq) + H2(aq) given the following information: Standard Entropy Values of Various Substance Substance So (J/mol • K) 60.9 Zn(s) HCl(aq) 56.5 130.58 H2(g) Zn2+(aq) -106.5 55.10 CI (aq)arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning


