
Concept explainers
Describe what is meant by each of the following reaction types, and illustrate with an example:
(a) nucleophilic substitution reaction: (b) electrophilic substitution reaction; (c) addition reaction;
(d) elimination reaction, (e) rearrangement reaction.
(a)
Interpretation:
The nucleophilic substitution reaction should be defined with example.
Concept introduction:
Nucleophilic substitution reaction describes the attack of the electron-rich group that is nucleophile on electron deficient groups that is electrophile.
Answer to Problem 1E
Nucleophilic substitution reaction is the type of reaction in which the nucleophile (electron rich species) attacks the electron-deficient carbon atom which is electrophilic.
Explanation of Solution
Nucleophilic substitution reaction is defined as an organic reaction which includes the attack of a nucleophile on electrophilic center along with the removal of the leaving group.
The example of the nucleophilic substitution reaction is,
In this reaction, chlorine of chloroethane is replaced by a hydroxyl group
(b)
Interpretation:
The electrophilic substitution reaction should be defined with example.
Concept introduction:
The electrophilic substitution reaction describes the displacement of functional group or hydrogen atom by an electron deficient group or electrophile.
Answer to Problem 1E
Electrophilic substitution reaction is defined as the organic reaction in which the electrophile replaces a functional group of a compound or hydrogen atom.
Explanation of Solution
Electrophilic substitution reaction is defined as the organic reaction which includes the replacement of functional group or
Example of electrophilic substitution reaction is,
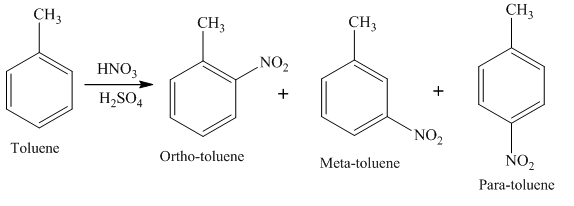
In this reaction, Toluene undergoes electrophilic substitution to form para nitrotoluene, meta nitrotoluene, and ortho nitrotoluene.
(c)
Interpretation:
The addition reaction should be defined with example.
Concept introduction:
The addition reaction describes the combination of two or more smaller molecules to form a larger molecule.
Answer to Problem 1E
Addition reaction is defined as the reaction in which two or more molecules combine to form a single and large molecule.
Explanation of Solution
The reaction of the addition of the two or more reactants that is A and B to produce a single product C is termed as addition reaction.
The example of addition reaction is,
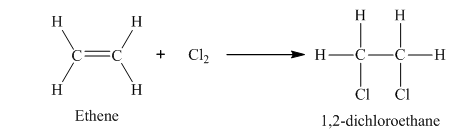
In this reaction, chlorine molecule combines with ethene to form 1, 2-dichloroethane.
(d)
Interpretation:
The elimination reaction should be defined with example.
Concept introduction:
The elimination reaction describes the removal of two substituents from the reactant molecule to form the product.
Answer to Problem 1E
Elimination reaction is the reaction by which the reactant molecule or compound breaks into two or more products.
Explanation of Solution
Elimination reaction is the type of reaction in which two substituents are removed from the reactant molecule to form the product. Generally, unsaturated compounds are formed in an elimination reaction.
The example of the elimination reaction is,
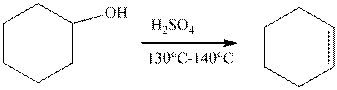
The reaction of cyclohexanol in the presence of
(e)
Interpretation:
The rearrangement reaction should be defined with example.
Concept introduction:
The rearrangement reaction describes the rearrangement of bonds in a molecule to form the product.
Answer to Problem 1E
It is the process of movement of bonds within a molecule to give rise to structural isomers.
Explanation of Solution
It is defined as a reaction in which an atom or a bond migrates from one atom in reactant molecule to adjacent atom to give rise to the product.
Example of rearrangement reaction is,
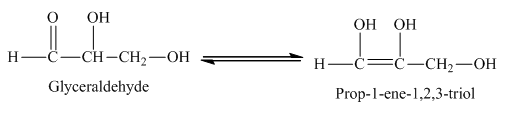
The glyceraldehyde undergoes rearrangement to form enediol.
Want to see more full solutions like this?
Chapter 27 Solutions
EBK GENERAL CHEMISTRY
Additional Science Textbook Solutions
Human Physiology: An Integrated Approach (8th Edition)
Organic Chemistry
Physical Universe
Campbell Biology: Concepts & Connections (9th Edition)
General, Organic, and Biological Chemistry - 4th edition
- Explain why this data led Rayleigh to look for and to discover Ar.arrow_forward5) Confidence interval. Berglund and Wichardt investigated the quantitative determination of Cr in high-alloy steels using a potentiometric titration of Cr(VI). Before the titration, samples of the steel were dissolved in acid and the chromium oxidized to Cr(VI) using peroxydisulfate. Shown here are the results (as %w/w Cr) for the analysis of a reference steel. 16.968, 16.922, 16.840, 16.883, 16.887, 16.977, 16.857, 16.728 Calculate the mean, the standard deviation, and the 95% confidence interval about the mean. What does this confidence interval mean?arrow_forwardIn the Nitrous Acid Test for Amines, what is the observable result for primary amines? Group of answer choices nitrogen gas bubbles form a soluble nitrite salt yellow oily layer of nitrosoaminearrow_forward
- 3. a. Use the MS to propose at least two possible molecular formulas. For an unknown compound: 101. 27.0 29.0 41.0 50.0 52.0 55.0 57.0 100 57.5 58.0 58.5 62.0 63.0 64.0 65.0 74.0 40 75.0 76.0 20 20 40 60 80 100 120 140 160 180 200 220 m/z 99.5 68564810898409581251883040 115.0 116.0 77404799 17417M 117.0 12.9 118.0 33.5 119.0 36 133 0 1.2 157.0 2.1 159.0 16 169.0 219 170.0 17 171.0 21.6 172.0 17 181.0 1.3 183.0 197.0 100.0 198.0 200. 784 Relative Intensity 2 2 8 ō (ppm) 6 2arrow_forwardSolve the structure and assign each of the following spectra (IR and C-NMR)arrow_forward1. For an unknown compound with a molecular formula of C8H100: a. What is the DU? (show your work) b. Solve the structure and assign each of the following spectra. 8 6 2 ō (ppm) 4 2 0 200 150 100 50 ō (ppm) LOD D 4000 3000 2000 1500 1000 500 HAVENUMBERI -11arrow_forward
- 16. The proton NMR spectral information shown in this problem is for a compound with formula CioH,N. Expansions are shown for the region from 8.7 to 7.0 ppm. The normal carbon-13 spec- tral results, including DEPT-135 and DEPT-90 results, are tabulated: 7 J Normal Carbon DEPT-135 DEPT-90 19 ppm Positive No peak 122 Positive Positive cus и 124 Positive Positive 126 Positive Positive 128 No peak No peak 4° 129 Positive Positive 130 Positive Positive (144 No peak No peak 148 No peak No peak 150 Positive Positive してしarrow_forward3. Propose a synthesis for the following transformation. Do not draw an arrow-pushing mechanism below, but make sure to draw the product of each proposed step (3 points). + En CN CNarrow_forwardShow work..don't give Ai generated solution...arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





