
Selected Solutions Manual For General Chemistry: Principles And Modern Applications
11th Edition
ISBN: 9780133387902
Author: Ralph H. Petrucci, F. Geoffrey Herring, Jeffry D. Madura, Carey Bissonnette
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 26, Problem 9E
What is the relationship, if any, between the molecules in each of the following pairs? The relationship may be any of identical structures, constitutional isomers, stereoisomers, or no relationship.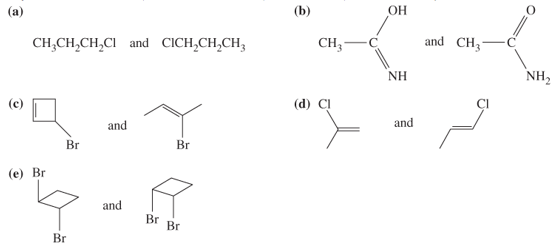
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Indicate the names of these compounds (if they exist).
0:
HỌC—NH
CH3CH2-CH2
N
Classify each of the following molecules as aromatic, antiaromatic, or nonaromatic.
NH
O aromatic
O antiaromatic
O nonaromatic
O aromatic
O antiaromatic
O nonaromatic
O aromatic
O antiaromatic
O nonaromatic
G
The conjugate base of alkanes is called alkides. Correct?.
Chapter 26 Solutions
Selected Solutions Manual For General Chemistry: Principles And Modern Applications
Ch. 26 - Prob. 1ECh. 26 - Draw a structural formula for each of the...Ch. 26 - Prob. 3ECh. 26 - Write structural formulas corresponding to these...Ch. 26 - Prob. 5ECh. 26 - Prob. 6ECh. 26 - Prob. 7ECh. 26 - Prob. 8ECh. 26 - What is the relationship, if any, between the...Ch. 26 - Prob. 10E
Ch. 26 - Prob. 11ECh. 26 - Prob. 12ECh. 26 - Identify the chiral carbon atoms, ¡f any, in the...Ch. 26 - Prob. 14ECh. 26 - Identify the chiral carbon atoms, ¡f any, in the...Ch. 26 - Prob. 16ECh. 26 - Prob. 17ECh. 26 - Prob. 18ECh. 26 - Prob. 19ECh. 26 - By name or formula, give one example of each of...Ch. 26 - Prob. 21ECh. 26 - Prob. 22ECh. 26 - Prob. 23ECh. 26 - Prob. 24ECh. 26 - Prob. 25ECh. 26 - Prob. 26ECh. 26 - Prob. 27ECh. 26 - Prob. 28ECh. 26 - Prob. 29ECh. 26 - Prob. 30ECh. 26 - Prob. 31ECh. 26 - Prob. 32ECh. 26 - Prob. 33ECh. 26 - Prob. 34ECh. 26 - Does each of the following names convey sufficient...Ch. 26 - Prob. 36ECh. 26 - Prob. 37ECh. 26 - Supply condensed structural formulas for the...Ch. 26 - Prob. 39ECh. 26 - Prob. 40ECh. 26 - Classify the carbon atoms in, a. methylbutane, and...Ch. 26 - Classity the carbon atoms in a....Ch. 26 - Prob. 43ECh. 26 - Draw Newman projections for the staggered and...Ch. 26 - Draw the most stable conformation for the molecule...Ch. 26 - Prob. 46ECh. 26 - Prob. 47ECh. 26 - Prob. 48ECh. 26 - Prob. 49ECh. 26 - Prob. 50ECh. 26 - Prob. 51ECh. 26 - Prob. 52ECh. 26 - Prob. 53ECh. 26 - Prob. 54ECh. 26 - Prob. 55ECh. 26 - Prob. 56ECh. 26 - Draw suitable structural formulas to show that...Ch. 26 - Which of the following pairs of molecules are...Ch. 26 - Prob. 59ECh. 26 - Prob. 60ECh. 26 - Name the following molecules with the appropriate...Ch. 26 - Name the following molecules with the appropriate...Ch. 26 - Name the following molecules with the appropriate...Ch. 26 - Prob. 64ECh. 26 - Draw the structure for each of the following. a....Ch. 26 - Prob. 66ECh. 26 - Prob. 67ECh. 26 - Prob. 68ECh. 26 - Prob. 69ECh. 26 - Prob. 70ECh. 26 - Prob. 71ECh. 26 - Prob. 72ECh. 26 - Prob. 73ECh. 26 - Prob. 74ECh. 26 - Supply condensed or structural formulas for the...Ch. 26 - Prob. 76IAECh. 26 - Prob. 77IAECh. 26 - Prob. 78IAECh. 26 - Prob. 79IAECh. 26 - Prob. 80IAECh. 26 - Combustion of a 0.1908 g sample of a compound gave...Ch. 26 - Prob. 82IAECh. 26 - In the monochiorination of hydrocarbons, a...Ch. 26 - A particular colorless organic liquid is known to...Ch. 26 - Prob. 85IAECh. 26 - Give the systematic names, including any...Ch. 26 - Prob. 87IAECh. 26 - Prob. 88IAECh. 26 - Levomethadyl acetate (shown below) is used in the...Ch. 26 - Thiamphenicol (shown below) is an antibacterial...Ch. 26 - Prob. 91IAECh. 26 - Prob. 92IAECh. 26 - Prob. 93IAECh. 26 - Prob. 94IAECh. 26 - Prob. 95IAECh. 26 - For each of the following molecules (a) draw the...Ch. 26 - Prob. 97FPCh. 26 - Prob. 98SAECh. 26 - Explain the important distinctions between each...Ch. 26 - Describe the characteristics of each of the...Ch. 26 - The compound isoheptane is best represented by the...Ch. 26 - Prob. 102SAECh. 26 - Prob. 103SAECh. 26 - Prob. 104SAECh. 26 - Assign configurations, R or S, to the chiral...Ch. 26 - Consider the following pairs of structures In each...Ch. 26 - Prob. 107SAECh. 26 - Prob. 108SAECh. 26 - Prob. 109SAECh. 26 - Prob. 110SAECh. 26 - Prob. 111SAE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Name these organic compounds: structure Br name CH3 CH3 ☐ ☐arrow_forwardHH H-C H -C-H HH Draw the Skeletal Structures & H Name the molecules HH H H H H-C-C-C-C-C-C-H HHH HHH H H HHHHHHH H-C-C-C-C-C-C-C-C-C-H HHHHH H H H Harrow_forwarddont provide AI solution .... otherwise i will give you dislikearrow_forward
- Name these organic compounds: structure name CH3 CH3 ☐ F F CH3 ☐ O Explanation Check 2025 McGraw Hill LLC. All Rights Reserved. Terms ofarrow_forwardClassify each of the following molecules as aromatic, antiaromatic, or nonaromatic. ZI NH Explanation Check O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic H O nonaromatic O aromatic O antiaromatic O nonaromatic ×arrow_forwardPart I. Draw the stepwise reaction mechanism of each product (a, b, c, d, e, f) HO HO OH НОН,С HO OH Sucrose HO CH₂OH H N N HO -H H -OH KMnO4, Heat H OH CH₂OH (d) Phenyl Osatriazole OH НОН,С HO HO + Glacial HOAC HO- HO CH₂OH OH HO Fructose (a) Glucose OH (b) H₂N HN (c) CuSO4-5H2O, ethanol H N N N HO ·H H OH H OH N CH₂OH OH (f) Phenyl Osazone H (e) Carboxy phenyl osatriazole Figure 2.1. Reaction Scheme for the Total Synthesis of Fine Chemicalsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License