
(a)
Interpretation:
The way in which the structure of the given compound differs from the structure of fats and oils has to be described.
Fats: Glycerol is a part of the structure of fat and is made up of three Carbon atoms. fats are made up of fatty acids, having a long carbon chain with one end of the chain possessing a carbonyl (COOH) groups.
Triglycerides: The major molecule which starts the structure of a triglyceride is glycerol. Glycerol is a three-carbon molecule with three hydroxyl groups on them. These hydroxyl groups are the sites of an ester reaction with three fatty acid molecules. The fatty acids can be different, and are long chains of carbons.
Formation of triglyceride:
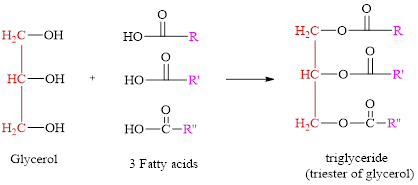
General structure of triglyceride is,
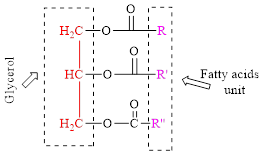
(b)
Interpretation:
The three products obtained when the given compound is hydrolyzed with aqueous Sodium hydroxide has to be drawn.
(c)
Interpretation:
Four compounds are obtained when the given compound is hydrolyzed with aqueous acid; all the possible structures have to be drawn.
Trending nowThis is a popular solution!

Chapter 26 Solutions
ORGANIC CHEMISTRYPKGDRL+MLCRL MDL
- The following chemical structure represents a molecule of what molecular formula?arrow_forwardWhich region(s) of the following phospholipid is/are hydrophobic? RO I hydro-water phobic-dislikes = Hydrophobic dislikes water ○ I only Il only I and III only II and IV only O II, III, and IV only III || IVarrow_forwardPredict the product of the following reactions: O 0= excess Х Кон ОН H+ H+ Iarrow_forward
- How many chiral centers/stereocenters are there in the following molecule? 1 2 3 4arrow_forwardWhich of these correspond to the molecule: 2,5-dimethylheptanearrow_forwardGiven the following data, determine the order of the reaction with respect to H2. H2(g) + 21Cl(g) → I2(g) + 2HCl(g) Experiment [H2] (torr) [ICI] (torr) Rate (M/s) 1 250 325 0.266 2 250 81 0.0665 3 50 325 0.266arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





