
(a)
Interpretation:
The working method of ion mobility spectrometry should be describes and analogies between gas-phase electrophoresis and capillary electrophoresis should be given.
Concept introduction:
Ion mobility spectrometry:
The electrophoretic mobility is given by the constant of proportionality between the electric field strength and speeds of the ion.
Capillary electrophoresis:
The movement of solute particle respect with the applied electric field is known as electrophoresis, this electrophoresis is produced in capillary tube is known as capillary electrophoresis.
(a)
Explanation of Solution
To describe the working method of ion mobility spectrometry.
The analyte are converted into gaseous ions by irradiation of analyte plus reagent gas like acetone in air with
The produced ions are passed through a tube, which has the grid with short voltage to plus the ions.
The ions are experience the constant electrical field in the grid. The ions are detected by the detectors, which the time they are drift.
The speed of the ion respect with the applied constant short voltage of the grid and friction force of the tube are noted.
Electrophoretic mobility is the constant of proportionality between the electric field strength and speeds of the ion.
Here, f is the friction coefficient.
From the above collected data are plugged in above equation to find the electrophoretic mobility of ion in electrophoresis, the ions are separated by resulted electrophoretic mobility of ions.
To give the analogies between gas-phase electrophoresis and capillary electrophoresis.
The drift time in the capillary electrophoresis same as the capillary electrophoresis.
The applied electrical field is a driving force of ions to migrate source to detector in capillary electrophoresis and same as in gas-phase electrophoresis.
The mobility of the ion in gas-phase electrophoresis and capillary electrophoresis is governed by charge to size ration.
The smaller size higher charge ions are have greater mobility.
The retarding force of the liquids and gases in gas-phase electrophoresis and capillary electrophoresis is caused by collision with medium.
The working method of ion mobility spectrometry was describes and analogies between gas-phase electrophoresis and capillary electrophoresis was given.
(b)
Interpretation:
The shape of curve in graph of plate number (vs) voltage in ion mobility spectrometry should be explained and the disadvantage of
Concept introduction:
Ion mobility spectrometry:
The electrophoretic mobility is given by the constant of proportionality between the electric field strength and speeds of the ion.
The peak width of the Ion mobility spectrometry is given by,
Where,
From the above equation the plate number is,
Where,
(b)
Explanation of Solution
To describe the working method of ion mobility spectrometry.
To draw the graph plate number (vs) voltage in ion mobility spectrometry.
Given,
Temperature
The give data and give formula are plugged in the spread sheet and apply the spread sheet formatted formula to calculate the required values and to prepare a graph of N (vs) Voltage
To take a new spread sheet and type the heading, which is shown in figure 1 and enter the observed values in the respective columns as pre heading.
The spread sheet format formulas are entered in column A same as shown in figure 1.
The values of plate number N respect with voltage are manipulated by the formula, which is already entered in spread sheet.
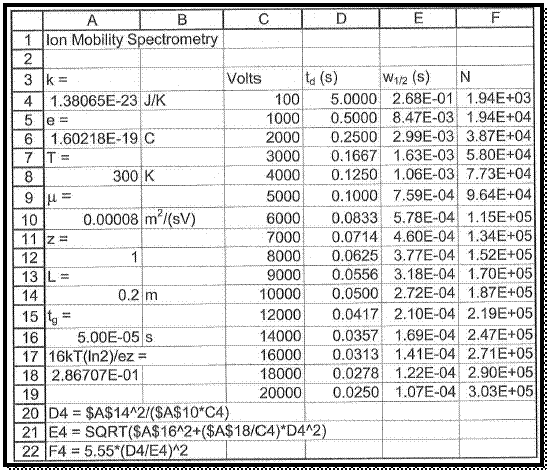
Figure 1
The graph is drawn by using values, which is in spread sheet.
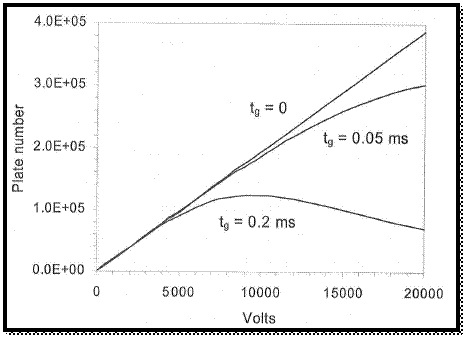
Figure 2
From the above graph, the applied voltage is increases the number of plats.
The broadening of peak is resulted by the decreasing of tome to diffusion and the initial peak width is increases with the increasing of gate open time of ion hence the plot number is decreased.
Therefore, the peak is not obtained in narrowed than the plus that admitted by the gate.
To give the disadvantage of
At the high voltage, the
The shape of curve in graph of plate number (vs) voltage in ion mobility spectrometry was explained and the disadvantage of
(c)
Interpretation:
The reason for the decreasing T increase N in ion mobility spectrometry should be given.
Concept introduction:
Ion mobility spectrometry:
The electrophoretic mobility is given by the constant of proportionality between the electric field strength and speeds of the ion.
The peak width of the Ion mobility spectrometry is given by,
Where,
From the above equation the plate number is,
Where,
(c)
Explanation of Solution
To give the reason for the decreasing T increase N in ion mobility spectrometry.
The peak width of the Ion mobility spectrometry is given by,
The equation for the plate number is,
From the above equation 1, the temperature is directly proportional peak width and in equation 2 the number of plates is indirectly proportional to the peak width. Therefore decreasing temperature is increasing number of plates because of broadening of peak is decreases with decreasing temperature.
The reason for the decreasing T increase N in ion mobility spectrometry was given.
(d)
Interpretation:
The theoretical plate number of given mobility spectrometry should be calculated.
Concept introduction:
Ion mobility spectrometry:
The electrophoretic mobility is given by the constant of proportionality between the electric field strength and speeds of the ion.
The peak width of the Ion mobility spectrometry is given by,
Where,
From the above equation the plate number is,
Where,
(d)
Answer to Problem 26.50P
The theoretical plate number is
Explanation of Solution
To calculate the theoretical plate number of given mobility spectrometry.
Given,
The equation for the plate number is,
The theoretical plate number is,
The calculated width of the peak and
The theoretical plate number is
The theoretical plate number of given ion mobility spectrometry was calculated.
(e)
Interpretation:
The resolution of the given two peaks of ion mobility spectrometry should be calculated.
Concept introduction:
Resolution:
The difference in the two peaks, which are obtained in the same experiment in same condition is given by resolution.
In the chromatographic separation, the resolution is,
Where,
(e)
Answer to Problem 26.50P
The resolution of the given two peaks of ion mobility spectrometry is
Explanation of Solution
To calculate the resolution of the given two peaks of ion mobility spectrometry.
Given,
Number of plates
Resolution of given two peaks is,
The difference in the apparent mobility of the ions, number of plates, and average apparent mobility are plugged in above equation to give the Resolution of given two peaks.
Resolution of given two peaks is
The resolution of the given two peaks of ion mobility spectrometry was calculated.
Want to see more full solutions like this?
Chapter 26 Solutions
Quantitative Chemical Analysis
- 5.arrow_forward6.arrow_forward0/5 alekscgi/x/sl.exe/1o_u-IgNglkr7j8P3jH-IQs_pBaHhvlTCeeBZbufuBYTi0Hz7m7D3ZcSLEFovsXaorzoFtUs | AbtAURtkqzol 1HRAS286, O States of Matter Sketching a described thermodynamic change on a phase diagram The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 3 pressure (atm) + 0- 0 5+ 200 temperature (K) 400 Explanation Check X 0+ F3 F4 F5 F6 F7 S 2025 McGraw Hill LLC All Rights Reserved. Terms of Use Privacy Center Accessibility Q Search LUCR + F8 F9 F10 F11 F12 * % & ( 5 6 7 8 9 Y'S Dele Insert PrtSc + Backsarrow_forward
- 5.arrow_forward9arrow_forwardalekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IQs_pBanHhvlTCeeBZbufu BYTI0Hz7m7D3ZS18w-nDB10538ZsAtmorZoFusYj2Xu9b78gZo- O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 3- 200 temperature (K) Explanation Chick Q Sowncharrow_forward
- 0+ aleksog/x/lsl.exe/1ou-lgNgkr7j8P3H-IQs pBaHhviTCeeBZbufuBYTOHz7m7D3ZStEPTBSB3u9bsp3Da pl19qomOXLhvWbH9wmXW5zm O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 Gab The temperature on a sample of pure X held at 0.75 atm and -229. °C is increased until the sample sublimes. The temperature is then held constant and the pressure is decreased by 0.50 atm. On the phase diagram below draw a path that shows this set of changes. F3 pressure (atm) 0- 0 200 Explanation temperature (K) Check F4 F5 ☀+ Q Search Chill Will an 9 ENG F6 F7 F8 F9 8 Delete F10 F11 F12 Insert PrtSc 114 d Ararrow_forwardx + LEKS: Using a phase diagram a X n/alekscgi/x/lsl.exe/10_u-IgNsikr7j8P3jH-IQs_pBan HhvlTCeeBZbufu BYTI0Hz7m7D3ZcHYUt80XL-5alyVpw ○ States of Matter Using a phase diagram to find a phase transition temperature or pressure Use the phase diagram of Substance X below to find the melting point of X when the pressure above the solid is 1.1 atm. pressure (atm) 16 08- solid liquid- 0 200 400 gas 600 temperature (K) Note: your answer must be within 25 °C of the exact answer to be graded correct. × 5arrow_forwardS: Using a phase diagram leksogi/x/sl.exe/1ou-IgNs kr 7j8P3jH-IQs_pBan HhvTCeeBZbufuBYTI0Hz7m7D3ZdHYU+80XL-5alyVp O States of Matter Using a phase diagram to find a phase transition temperature or pressure se the phase diagram of Substance X below to find the boiling point of X when the pressure on the liquid is 1.6 atm. pressure (atm) 32- 16- solid liquid 0. gas 100 200 temperature (K) 300 Note: your answer must be within 12.5 °C of the exact answer to be graded correct. 10 Explanation Check § Q Search J 2025 McGraw Hill LLC. All Rights Researrow_forward
- 151.2 254.8 85.9 199.6 241.4 87.6 242.5 186.4 155.8 257.1 242.9 253.3 256.0 216.6 108.7 239.0 149.7 236.4 152.1 222.7 148.7 278.2 268.7 234.4 262.7 283.2 143.6 QUESTION: Using this group of data on salt reduced tomato sauce concentration readings answer the following questions: 1. 95% Cl Confidence Interval (mmol/L) 2. [Na+] (mg/100 mL) 3. 95% Na+ Confidence Interval (mg/100 mL)arrow_forwardResults Search Results Best Free Coursehero Unloc xb Success Confirmation of Q x O Google Pas alekscgi/x/lsl.exe/1o_u-IgNslkr 7j8P3jH-IQs_pBanHhvlTCeeBZbufu BYTI0Hz7m7D3ZcHYUt80XL-5alyVpwDXM TEZayFYCavJ17dZtpxbFD0Qggd1J O States of Matter Using a phase diagram to find a phase transition temperature or pressure Gabr 3/5 he pressure above a pure sample of solid Substance X at 101. °C is lowered. At what pressure will the sample sublime? Use the phase diagram of X below to nd your answer. pressure (atm) 24- 12 solid liquid gas 200 400 temperature (K) 600 ote: your answer must be within 0.15 atm of the exact answer to be graded correct. atm Thanation Check © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center I Q Search L³ ملةarrow_forward301.7 348.9 193.7 308.6 339.5 160.6 337.7 464.7 223.5 370.5 326.6 327.5 336.1 317.9 203.8 329.8 221.9 331.7 211.7 309.6 223.4 353.7 334.6 305.6 340.0 304.3 244.7 QUESTION: Using this group of data on regular tomato sauce concentration readings answer the following questions: 1. 95% Cl Confidence Interval (mmol/L) 2. [Na+] (mg/100 mL) 3. 95% Na+ Confidence Interval (mg/100 mL)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





