
Organic Chemistry
5th Edition
ISBN: 9780078021558
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 26, Problem 26.13P
Draw the products formed when each
a.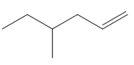 b.
b. 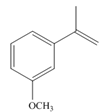 c.
c. 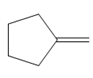
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Consider the structures below to answer the following questions.
A. Indicate the most acidic hydrogens in each of the molecules.
OH
CH-H
CH₂C-H
H&C
མིངྒཱའི
B. Rank the molecules above in order of increasing acidity (least acidic to most acidic).
a.
III, II, I
b. II, III, I
C.
I, II, III
d. II, I, III
Consider the reaction below to answer the following questions.
H
H+
A
B
CH₂OH
5% NaOCH, CH₂OH
A. Which carbonyl compound functions as the electrophile in this reaction?
B. Draw the structure of the enolate ion that is generated during the course of this reaction.
C. This reaction is an example of:
a. a mixed Claisen condensation.
b.
C.
d.
a Dieckman condensation.
a Michael reaction.
a mixed aldol reaction.
Give the major organic product(s) of each of the following reactions or sequences of reactions. Show all
relevant stereochemistry. [two only]
CH3O
(11
HC-C-C-CH3
A.
CH3
12. NaOH
Chapter 26 Solutions
Organic Chemistry
Ch. 26 - Prob. 26.1PCh. 26 - Prob. 26.2PCh. 26 - Prob. 26.3PCh. 26 - Prob. 26.4PCh. 26 - Prob. 26.5PCh. 26 - Prob. 26.6PCh. 26 - Prob. 26.7PCh. 26 - Problem 26.8
What starting materials are needed to...Ch. 26 - Prob. 26.9PCh. 26 - Problem 26.10
What reagents are needed to convert...
Ch. 26 - Problem 26.11
What product is formed when each...Ch. 26 - Prob. 26.12PCh. 26 - Problem 26.13
Draw the products formed when each...Ch. 26 - Problem 26.14
What products are formed when ...Ch. 26 - Problem 26.15
Draw the product formed from...Ch. 26 -
What product is formed by ring-closing metathesis...Ch. 26 - Problem 26.17
What starting material is needed to...Ch. 26 - 26.18 In addition to organic halides, alkyl...Ch. 26 - 26.19 What product is formed by ring-closing...Ch. 26 - 26.20 Draw the products formed in each...Ch. 26 - What organic halide is needed to convert lithium...Ch. 26 - 26.22 How can you convert ethynylcyclohexane to...Ch. 26 - 26.23 What compound is needed to convert styrene...Ch. 26 - 26.24 What steps are needed to convert to octane...Ch. 26 - Prob. 26.25PCh. 26 - Prob. 26.26PCh. 26 - 26.27 Draw the products (including stereoisomers)...Ch. 26 - 26.28 Treatment of cyclohexene with and forms...Ch. 26 - Prob. 26.29PCh. 26 - 26.30 What starting material is needed to prepare...Ch. 26 - Prob. 26.31PCh. 26 - Prob. 26.32PCh. 26 - When certain cycloalkenes are used in metathesis...Ch. 26 - 26.34 Draw the products formed in each reaction.
...Ch. 26 - Prob. 26.35PCh. 26 - Draw a stepwise mechanism for the following...Ch. 26 - Sulfur ylides, like the phosphorus ylides of...Ch. 26 - Although diazomethane is often not a useful...Ch. 26 - Prob. 26.39PCh. 26 - Prob. 26.40PCh. 26 - Prob. 26.41PCh. 26 - Prob. 26.42PCh. 26 - 26.43 Devise a synthesis of each compound using a...Ch. 26 - 26.44 Devise a synthesis of each compound from...Ch. 26 - 26.45 Devise a synthesis of each compound from...Ch. 26 - 26.46 Devise a synthesis of each substituted...Ch. 26 - Biaryls, compounds containing two aromatic rings...Ch. 26 - Prob. 26.48PCh. 26 - 26.49 Draw the product formed from the...Ch. 26 - Prob. 26.50PCh. 26 - 26.51 Devise a synthesis of each of the following...Ch. 26 - Prob. 26.52PCh. 26 - 26.53 The following conversion, carried out in the...Ch. 26 - Prob. 26.54PCh. 26 - 26.55 Dimethyl cyclopropanes can be prepared by...Ch. 26 - Prob. 26.56P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Diethyl malonate can be prepared by the following reaction sequence. Draw the structures of each of the missing intermediates in the boxes provided. 17 1. Br PBr H&C OH 2 H₂O CH3CH₂OH На NaCN H₂SO4 NC. CH CH₂OH на H₂O, heat CH₂ OCHCH3 ཝསི། ཡིཀྑཱམུདྡྷནྟ CH₂ OEtarrow_forwardThe reaction of a carboxylic acid with an alcohol in the presence of acid is termed Fischer esterification. OH + CH₂OH На B C A. The nucleophile in this reaction is B. Compound C functions as a. a base scavenger b. a solvent C. a catalyst d. a neutralizer C. Fischer esterification is an example of: . a. nucleophilic acyl addition b. nucleophilic acyl substitution c. nucleophilic acyl elimination d. nucleophilic acyl rearrangement in this reaction.arrow_forwardA highly useful and general method for the synthesis of alcohols is the addition of Grignard reagents to carbonyl compounds. Show what Grignard reagent and what carbonyl compound you would start with to prepare each alcohol below. List all possibilities. OH C-CH2CH3 CH3arrow_forward
- Rank the following groups of compounds from most acidic (1) to least acidic (4). Place the number corresponding to the compound's relative rank in the blank below the structure. NO2 a. b. NO2 NO2 CH,CH,CH,CH,OH. CHCHCH-CHOH. CH-CH-CH,CH;OH CH-CHCH-CH-OH OH OH CH₂OH COH ဒီ ပုံ ပုံ H&C CN CN ĆNarrow_forwardGiven the major organic product(s) of each of the following reactions. If none is predicted, write "N.R." answer] a. CHỊCH, CHẤT AIC13 H b. 0 Cl₂ HC- NHOCH3 FeCl3arrow_forwardGive the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry [2 ONLY]. A -CH2COOH 1. LIAIH THF, heat 2 HO B. C. CH₂Br Br 1. NaCN, acetone 2 H₂O, heat 1. Mg ether 3 HO Z CO₂arrow_forward
- What is the order of increasing acidity for the following compounds? (least to most) [2 ONLY] A. COOH COOH COOH COOH 6666 a. IV, I, III, II b. III, II, I, IV с. II, III, I, IV d. III, II, IV, I slingoros CH3 IV woled noise bizarrow_forwardWith this, please answer the following questions: 1.) draw the structure of the electrophilic intermediate in this reaction. 2.) what is the role of the AlCl3 in the reaction 3.) write the complete stepwise mechanism for this reaction. Show all electron flow with arrows and include all intermediate structuresarrow_forwardConsider the data below to answer the following questions. Cyanohydrins are important intermediates in the synthesis of a-hydroxycarboxylic acids from ketones and aldehydes. The nitrile functional group can be hydrolyzed by aqueous acid to yield a carboxylic acid. Nitriles can also be hydrolyzed to carboxylic acids using aqueous base. Unfortunately, when a cyanohydrin is treated with aqueous base the original carbonyl compound is isolated. HO H HCEN H-3- HO' NaOH HO cyanohychin a. a nucleophilic substitution b. an electrophilic addition C10 OH CH-COOH A. The reaction of an aldehyde with hydrogen cyanide is an example of + NaCN + H₂O reaction. H- C. an electrophilic substitution d. a nucleophilic addition B. Identify the electrophile in the reaction of benzaldehyde with hydrogen cyanide.arrow_forward
- Refer to the data below to answer the following questions: The octapeptide saralasin is a specific antagonist of angiotensin II. A derivative of saralasin is used therapeutically as an antihypertensive. Amino acid analysis of saralasin show the presence of the following amino acids: Ala, Arg, His, Pro, Sar, Tyr, Val, Val A. Sar is the abbreviation for sarcosine, N-methyl aminoethanoic acid. Draw the structure of sarcosine. B. N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Tyr-Val-His Sar-Arg-Val His-Pro-Ala Val-Tyr-Val Arg-Val-Tyr What is the structure of saralasin?arrow_forwardGive the major organic product(s) of each of the following reactions or sequences of reactions. Show all relevant stereochemistry.[4 only] CH3 A. B. HNO H₂Pt H₂SO4 hano NaN 1. LIAH ether Br 4 2 H₂O C. D. E. CH3CH2-CH2CH3 + HCl Br NH₂ CH3 ON CH-CH3 Br HNOZ CUCI 11,504 HC) 1. HNO H SO NH₂ 2 UMarrow_forwardConsider the Grignard reaction below to answer the following questions. A Mgar 1. ether + MyC CH3 2H3O C B a. The electrophile in this reaction is: b. The nucleophile in this reaction is: c. The alcohol product can be classified as a: a. 1° alcohol b. 2° alcohol C. 3° alcohol d. 4° alcohol HO CH3 CHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY