
Concept explainers
a)
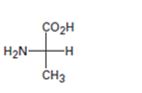
Interpretation:
The Fischer projection formula is to be converted in to a tetrahedral representation and its configuration as R or S is to be assigned.
Concept introduction:
In Fischer projection formula, a tetrahedral carbon is represented by two crossed lines. The horizontal line represents bonds coming out of the page and vertical lines represent bonds moving in to the page.
For assigning R or S configuration, the four groups attached to the chiral center are arranged in the order of priority by applying sequence rules. The molecule is then oriented in such a way that the group of lowest priority points away from the viewer. If the arrangement of highest priority to second highest priority to third highest priority is clockwise then R configuration is assigned. If the arrangement of highest priority to second highest priority to third highest priority is counterclockwise then S configuration is assigned.
To covert:
The Fischer projection formula in to a tetrahedral representation and to assign its configuration as R or S.
b)
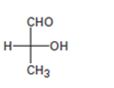
Interpretation:
The Fischer projection formula is to be converted in to a tetrahedral representation and its configuration as R or S is to be assigned.
Concept introduction:
In Fischer projection formula, a tetrahedral carbon is represented by two crossed lines. The horizontal line represents bonds coming out of the page and vertical lines represent bonds moving in to the page.
For assigning R or S configuration, the four groups attached to the chiral center are arranged in the order of priority by applying sequence rules. The molecule is then oriented in such a way that the group of lowest priority points away from the viewer. If the arrangement of highest priority to second highest priority to third highest priority is clockwise then R configuration is assigned. If the arrangement of highest priority to second highest priority to third highest priority is counterclockwise then S configuration is assigned.
To covert:
The Fischer projection formula in to a tetrahedral representation and to assign its configuration as R or S.
c)
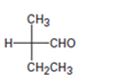
Interpretation:
The Fischer projection formula is to be converted in to a tetrahedral representation and its configuration as R or S is to be assigned.
Concept introduction:
In Fischer projection formula, a tetrahedral carbon is represented by two crossed lines. The horizontal line represents bonds coming out of the page and vertical lines represent bonds moving in to the page.
For assigning R or S configuration, the four groups attached to the chiral center are arranged in the order of priority by applying sequence rules. The molecule is then oriented in such a way that the group of lowest priority points away from the viewer. If the arrangement of highest priority to second highest priority to third highest priority is clockwise then R configuration is assigned. If the arrangement of highest priority to second highest priority to third highest priority is counterclockwise then S configuration is assigned.
To covert:
The Fischer projection formula in to a tetrahedral representation and to assign its configuration as R or S.
Trending nowThis is a popular solution!

Chapter 25 Solutions
ORGANIC CHEMISTRY-EBOOK>I<
- A 2-step reaction has the following mechanism: | 1. (fast) R2 R+R 2. (slow) R+Q K₂ P k_1 What series does it have? (A). v= - = (k + k1 − k-1)[R2][Q] (B). v=-k₁[R₂] + k₁[R]² - k₂[R][Q] (C). v=k₂[R]²[Q]² (D). v = k[R₂]1/2[Q]arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forward
- Steps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forward
- Steps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardLabel the α and ẞ carbons in each alkyl halide. Draw all possible elimination products formed when each alkyl halide is treated with K-OC(CH3), b. ان Brarrow_forwardSuppose a reaction has the following mechanism:A + B → C + D C + C → F F + B → A + A + GIt is known that C is a reaction intermediate. Of the following options, indicate which are true:1. The overall reaction could be 3B → 2D + G.2. A could be a catalyst.3. C is the only intermediate that can exist.arrow_forward
- Steps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forward
