
ORGANIC CHEMISTRY-NEXTGEN+BOX (2 SEM.)
4th Edition
ISBN: 9781119761068
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2.5, Problem 2.9P
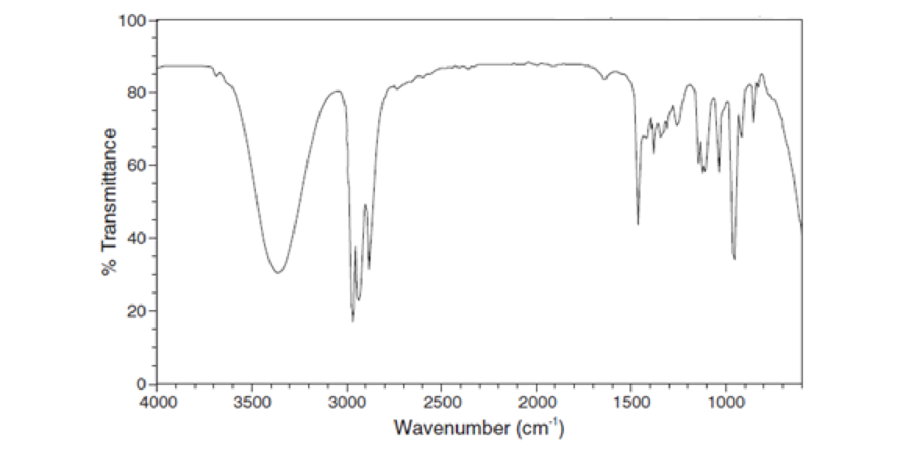
For each IR spectrum below, identify whether it is consistent with the structure of an alcohol, a
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
None
None
4. Draw and label all possible isomers for [M(py)3(DMSO)2(CI)] (py = pyridine, DMSO
dimethylsulfoxide).
Chapter 2 Solutions
ORGANIC CHEMISTRY-NEXTGEN+BOX (2 SEM.)
Ch. 2.3 - For each of the following compounds, determine...Ch. 2.3 - For each of the following compounds, determine...Ch. 2.3 - For each of the following compounds, determine...Ch. 2.3 - For each of the following compounds, determine...Ch. 2.3 - The following compound has three carbonyl groups....Ch. 2.4 - Predict which of the following C=C bonds will...Ch. 2.4 - The C=C bond in the following compound produces an...Ch. 2.5 - For each IR spectrum below, identify whether it is...Ch. 2.5 - For each IR spectrum below, identify whether it is...Ch. 2.5 - For each IR spectrum below, identify whether it is...
Ch. 2.5 - For each IR spectrum below, identify whether it is...Ch. 2.5 - For each IR spectrum below, identify whether it is...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.6 - Prob. 2.22P
Additional Science Textbook Solutions
Find more solutions based on key concepts
5. Distinguish between the anatomical neck and the surgical neck of the humerus. Name the proximal and distal p...
Principles of Anatomy and Physiology
11.57 Draw the cis and trans isomers for each of the following: (11.6)
a. 2-pentene
b. 3-hexene
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Using the pKa values listed in Table 15.1, predict the products of the following reactions:
Organic Chemistry (8th Edition)
Why are the top predators in food chains most severely affected by pesticides such as DDT?
Campbell Essential Biology (7th Edition)
Write an electron configuration for each element and the corresponding Lewis structure. Indicate which electron...
Introductory Chemistry (6th Edition)
Choose the best answer to each of the following. Explain your reasoning. When the ultraviolet light from hot st...
Cosmic Perspective Fundamentals
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The emission data in cps displayed in Table 1 is reported to two decimal places by the chemist. However, the instrument output is shown in Table 2. Table 2. Iron emission from ICP-AES Sample Blank Standard Emission, cps 579.503252562 9308340.13122 Unknown Sample 343.232365741 Did the chemist make the correct choice in how they choose to display the data up in Table 1? Choose the best explanation from the choices below. No. Since the instrument calculates 12 digits for all values, they should all be kept and not truncated. Doing so would eliminate significant information. No. Since the instrument calculates 5 decimal places for the standard, all of the values should be limited to the same number. The other decimal places are not significant for the blank and unknown sample. Yes. The way Saman made the standards was limited by the 250-mL volumetric flask. This glassware can report values to 2 decimal places, and this establishes our number of significant figures. Yes. Instrumental data…arrow_forwardSteps and explanation pleasearrow_forwardSteps and explanation to undertand concepts.arrow_forward
- Nonearrow_forward7. Draw a curved arrow mechanism for the following reaction. HO cat. HCI OH in dioxane with 4A molecular sievesarrow_forwardTry: Convert the given 3D perspective structure to Newman projection about C2 - C3 bond (C2 carbon in the front). Also, show Newman projection of other possible staggered conformers and circle the most stable conformation. Use the template shown. F H3C Br Harrow_forward
- Nonearrow_forward16. Consider the probability distribution p(x) = ax", 0 ≤ x ≤ 1 for a positive integer n. A. Derive an expression for the constant a, to normalize p(x). B. Compute the average (x) as a function of n. C. Compute σ2 = (x²) - (x)², the variance of x, as a function of n.arrow_forward451. Use the diffusion model from lecture that showed the likelihood of mixing occurring in a lattice model with eight lattice sites: Case Left Right A B C Permeable Barrier → and show that with 2V lattice sites on each side of the permeable barrier and a total of 2V white particles and 2V black particles, that perfect de-mixing (all one color on each side of the barrier) becomes increasingly unlikely as V increases.arrow_forward
- 46. Consider an ideal gas that occupies 2.50 dm³ at a pressure of 3.00 bar. If the gas is compressed isothermally at a constant external pressure so that the final volume is 0.500 dm³, calculate the smallest value Rest can have. Calculate the work involved using this value of Rext.arrow_forwardNonearrow_forward2010. Suppose that a 10 kg mass of iron at 20 C is dropped from a heigh of 100 meters. What is the kinetics energy of the mass just before it hits the ground, assuming no air resistance? What is its speed? What would be the final temperature of the mass if all the kinetic energy at impact is transformed into internal energy? The molar heat capacity of iron is Cpp = 25.1J mol-¹ K-1 and the gravitational acceleration constant is 9.8 m s¯² |arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY