
Organic Chemistry As a Second Language: Second Semester Topics
4th Edition
ISBN: 9781119110651
Author: David R. Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2.5, Problem 2.9P
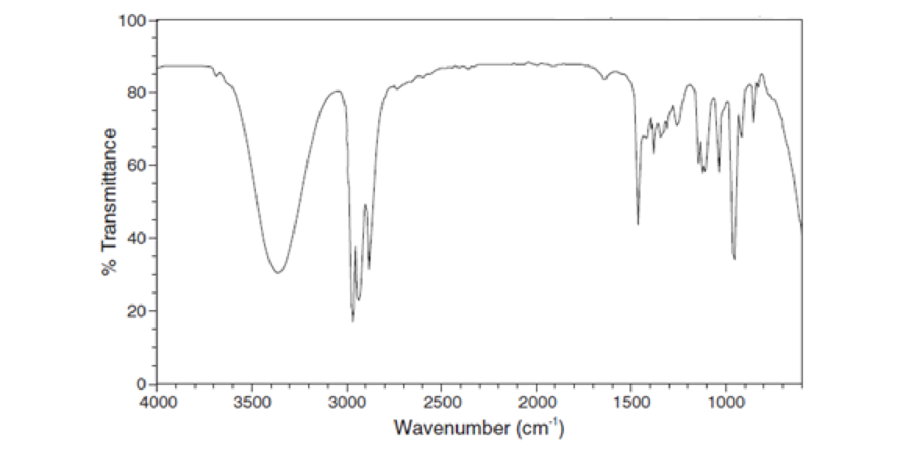
For each IR spectrum below, identify whether it is consistent with the structure of an alcohol, a
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the reaction mechanism to predict the product of the transformation below:
N
H
?
H₂O
Provide steps and explanation.
Steps and explanation.
Chapter 2 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
Ch. 2.3 - For each of the following compounds, determine...Ch. 2.3 - For each of the following compounds, determine...Ch. 2.3 - For each of the following compounds, determine...Ch. 2.3 - For each of the following compounds, determine...Ch. 2.3 - The following compound has three carbonyl groups....Ch. 2.4 - Predict which of the following C=C bonds will...Ch. 2.4 - The C=C bond in the following compound produces an...Ch. 2.5 - For each IR spectrum below, identify whether it is...Ch. 2.5 - For each IR spectrum below, identify whether it is...Ch. 2.5 - For each IR spectrum below, identify whether it is...
Ch. 2.5 - For each IR spectrum below, identify whether it is...Ch. 2.5 - For each IR spectrum below, identify whether it is...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.6 - Prob. 2.22P
Additional Science Textbook Solutions
Find more solutions based on key concepts
5. Distinguish between the anatomical neck and the surgical neck of the humerus. Name the proximal and distal p...
Principles of Anatomy and Physiology
11.57 Draw the cis and trans isomers for each of the following: (11.6)
a. 2-pentene
b. 3-hexene
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Using the pKa values listed in Table 15.1, predict the products of the following reactions:
Organic Chemistry (8th Edition)
Why are the top predators in food chains most severely affected by pesticides such as DDT?
Campbell Essential Biology (7th Edition)
Write an electron configuration for each element and the corresponding Lewis structure. Indicate which electron...
Introductory Chemistry (6th Edition)
Choose the best answer to each of the following. Explain your reasoning. When the ultraviolet light from hot st...
Cosmic Perspective Fundamentals
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Provide steps and explanation please.arrow_forwardDraw a structural formula for the major product of the acid-base reaction shown. H 0 N + HCI (1 mole) CH3 N' (1 mole) CH3 You do not have to consider stereochemistry. ● • Do not include counter-ions, e.g., Na+, I, in your answer. . In those cases in which there are two reactants, draw only the product from 989 CH3 344 ? [Farrow_forwardQuestion 15 What is the major neutral organic product for the following sequence? 1. POCI₂ pyridine ? 2. OsO4 OH 3. NaHSO Major Organic Product ✓ OH OH 'OH OH 'OH 'CIarrow_forward
- Could you please solve the first problem in this way and present it similarly but color-coded or step by step so I can understand it better? Thank you!arrow_forwardCould you please solve the first problem in this way and present it similarly but color-coded or step by step so I can understand it better? Thank you!arrow_forwardCould you please solve the first problem in this way and present it similarly but (color-coded) and step by step so I can understand it better? Thank you! I want to see what they are doingarrow_forward
- Can you please help mne with this problem. Im a visual person, so can you redraw it, potentislly color code and then as well explain it. I know im given CO2 use that to explain to me, as well as maybe give me a second example just to clarify even more with drawings (visuals) and explanations.arrow_forwardPart 1. Aqueous 0.010M AgNO 3 is slowly added to a 50-ml solution containing both carbonate [co32-] = 0.105 M and sulfate [soy] = 0.164 M anions. Given the ksp of Ag2CO3 and Ag₂ soy below. Answer the ff: Ag₂ CO3 = 2 Ag+ caq) + co} (aq) ksp = 8.10 × 10-12 Ag₂SO4 = 2Ag+(aq) + soy² (aq) ksp = 1.20 × 10-5 a) which salt will precipitate first? (b) What % of the first anion precipitated will remain in the solution. by the time the second anion starts to precipitate? (c) What is the effect of low pH (more acidic) condition on the separate of the carbonate and sulfate anions via silver precipitation? What is the effect of high pH (more basic)? Provide appropriate explanation per answerarrow_forwardPart 4. Butanoic acid (ka= 1.52× 10-5) has a partition coefficient of 3.0 (favors benzene) when distributed bet. water and benzene. What is the formal concentration of butanoic acid in each phase when 0.10M aqueous butanoic acid is extracted w❘ 25 mL of benzene 100 mL of a) at pit 5.00 b) at pH 9.00arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY