
Concept explainers
(a)
Interpretation:
Whether the statement “about 50% of TAGs undergo complete hydrolysis in the stomach” concerning to triacylglycerol(TAG) digestion is true or false has to be determined.
Concept introduction:
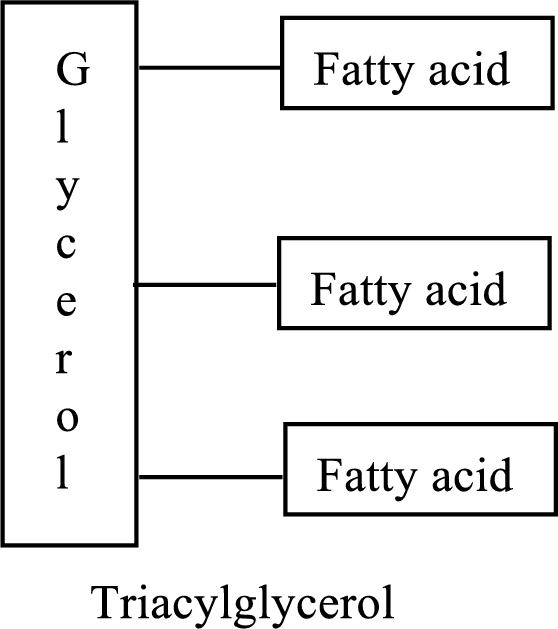
Triacylglycerols are lipid molecules which constitute around 98% of the total dietary lipids. These lipid molecules undergo digestion/breakdown into simpler forms in the
(b)
Interpretation:
Whether the statement “cholecystokinin is the chemical name for bile” concerning to triacylglycerol(TAG) digestion is true or false has to be determined.
Concept introduction:
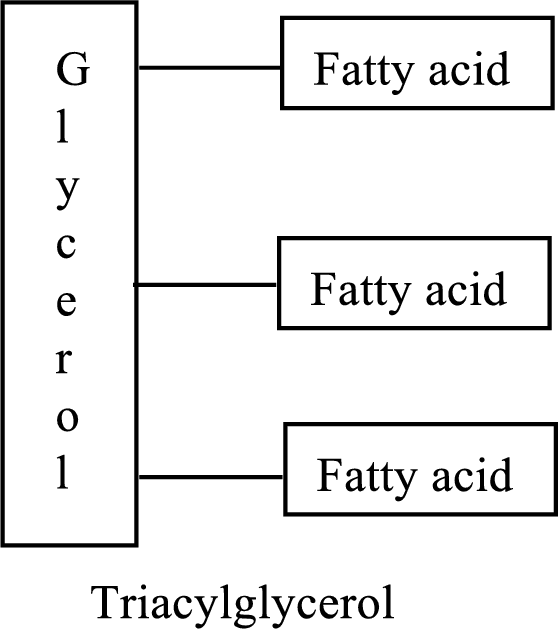
Triacylglycerols are lipid molecules which constitute around 98% of the total dietary lipids. These lipid molecules undergo digestion/breakdown into simpler forms in the digestive system and are later absorbed into the bloodstream. In the bloodstream, the hydrolysis products of triacylglycerols (fatty acids and glycerol) are absorbed by the body cells and are either broken down into acetyl CoA or stored as lipids for future use. Fats/Lipids are a richer source of energy compared to carbohydrates. While carbohydrates provide energy for immediate use, lipids provide energy for long term or future use. The structure of triacylglycerols is as follows:
(c)
Interpretation:
Whether the statement “most TAGs usually enter the small intestine in the form of monoacylglycerols” concerning to triacylglycerol(TAG) digestion is true or false has to be determined.
Concept introduction:
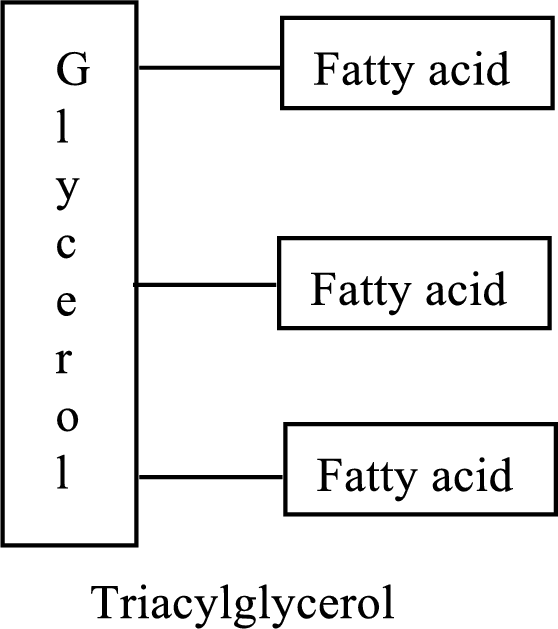
Triacylglycerols are lipid molecules which constitute around 98% of the total dietary lipids. These lipid molecules undergo digestion/breakdown into simpler forms in the digestive system and are later absorbed into the bloodstream. In the bloodstream, the hydrolysis products of triacylglycerols (fatty acids and glycerol) are absorbed by the body cells and are either broken down into acetyl CoA or stored as lipids for future use. Fats/Lipids are a richer source of energy compared to carbohydrates. While carbohydrates provide energy for immediate use, lipids provide energy for long term or future use. The structure of triacylglycerols is as follows:
(d)
Interpretation:
Whether the statement “chyme produced in the stomach settles to the bottom of the stomach” concerning to triacylglycerol(TAG) digestion is true or false has to be determined.
Concept introduction:
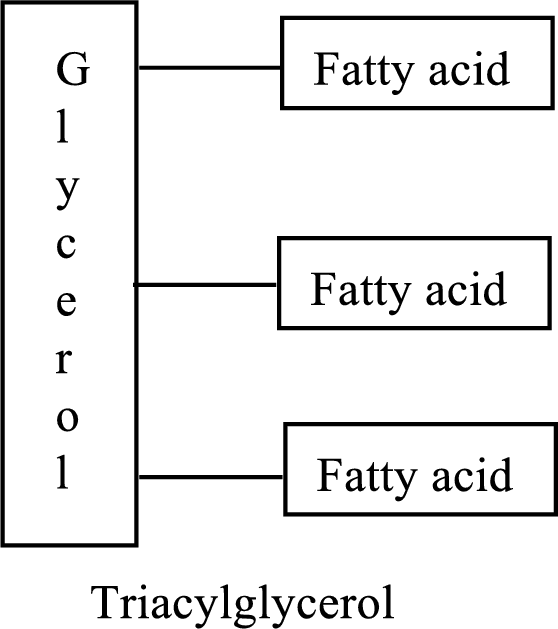
Triacylglycerols are lipid molecules which constitute around 98% of the total dietary lipids. These lipid molecules undergo digestion/breakdown into simpler forms in the digestive system and are later absorbed into the bloodstream. In the bloodstream, the hydrolysis products of triacylglycerols (fatty acids and glycerol) are absorbed by the body cells and are either broken down into acetyl CoA or stored as lipids for future use. Fats/Lipids are a richer source of energy compared to carbohydrates. While carbohydrates provide energy for immediate use, lipids provide energy for long term or future use.
The structure of triacylglycerols is as follows:
Want to see the full answer?
Check out a sample textbook solution
Chapter 25 Solutions
General, Organic, And Biological Chemistry, Hybrid (with Owlv2 Quick Prep For General Chemistry Printed Access Card)
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forward
- Synthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forward
- Indicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forward
- Question 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forwardIndicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are mixed with sodium ethoxide in ethanol.arrow_forwardIndicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are reacted with sodium ethoxide in ethanol.arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning




