
Interpretation:
In the structures of the given compounds, the carbon atoms that are expected to be labeled with
Concept introduction:
The isotopically labeled carbons in various compounds that are synthesized biologically enriched with
The organic material are dated using radioactive isotope of carbon
Answer to Problem 17P
Solution:
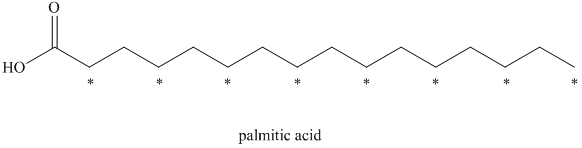
a) There are
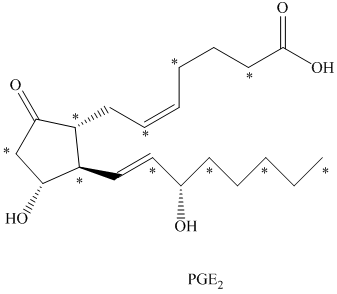
b) There are
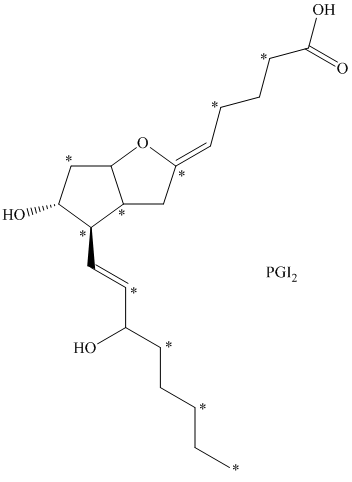
c) There are
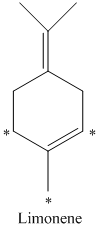
d) There are
e) There are
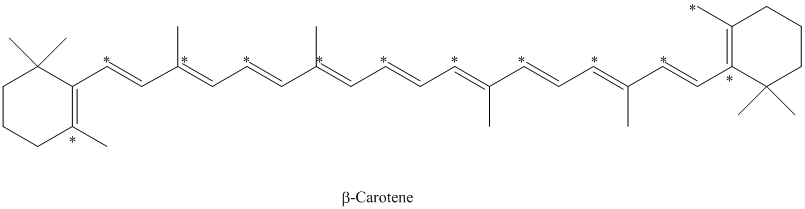
Explanation of Solution
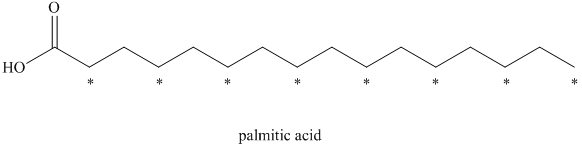
a) The given compound is Palmitic acid.
The isotopically labeled carbon atoms in palmitic acid synthesized from the acetate enriched with
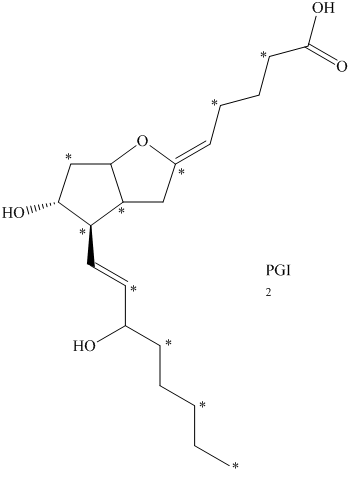
b) The given compound is
The isotopically labeled carbon atoms in
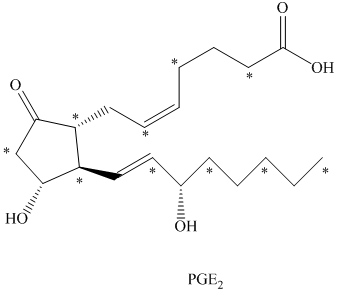
c) The given compound is
The isotopically labeled carbon atoms in
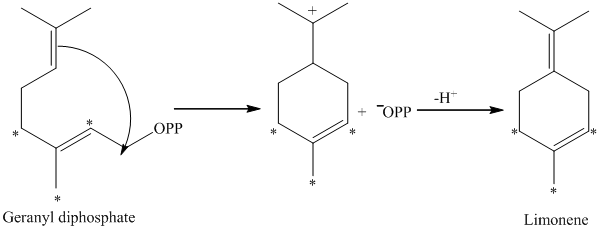
d) The given compound is limonene.
It is synthesized by the geranyl diphosphate that is further synthesized from dimethylallyl phosphate and isopentenyl diphosphate. The isotopically labeled carbon atoms in limonene enriched with
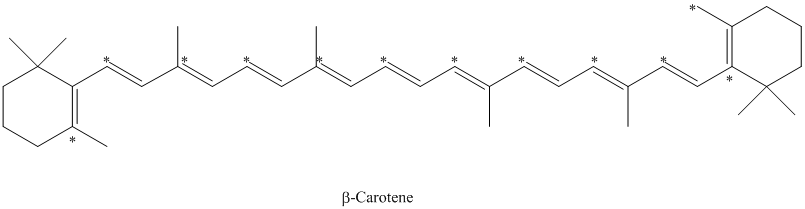
e) The given compound is
The isotopically labeled carbon atoms in
Want to see more full solutions like this?
Chapter 25 Solutions
ORGANIC CHEMISTRY (LOOSELEAF)-PACKAGE
- Refer to the monosaccharides below to answer each of the following questions: CH2OH CHO CH₂OH CHZOH 0 H OH 0 0 HO H H OH HO H HO H H OH HO H CHZOH H OH HO H HO H CHZOH CHZOH CH3 a Sorbose b. Rhamnose c. Erythrulose d. Xylulose Classify each sugar by type; for example, glucose is an aldohexose. A. Xylulose is B. Erythrulose is C. Sorbose is D. Rhamnose isarrow_forwardRefer to the sugars below to answer the following questions. Choose the sugar that best fits each escription and place the letter of the sugar in the blank to the left of the description. There is only one orrect answer for each question, but sugars may be used more than once. CH₂OH 0 CHO HO H CHO CH₂OH HO H HO H HO H H OH HH OH OH H OH H OH HO H CH₂OH H OH CH₂OH CH₂OH CH₂OH a (-)-tagatose b. (+) gulose c. (-)-erythrose d (-)-n bulos A. ARCD a D-ketohexose B. C. D. oxidizes to an optically inactive aldaric acid a dextrorotary hexose a ketose with two chirality centersarrow_forwardDraw the structure of the aldol, self condensation product for each of the following compounds if a compound does not undergo aldol self condensation explain why it does notarrow_forward
- Show how each of the following transformations might be best accomplished. More than one step may required. Show all reagents and all intermediate structures. [4 only] CH3 A. CH CH2 C Br CH3 B OH only source of carbon CH3 CH CH2 C NHz CH 3 Harrow_forward. Choose a structure from the list provided below that best fits each of the following escriptions. Place the letter of the structure in the blank to the left of the description. There is nly one correct answer for each question. starch HO CH₂OH b. cellulose d. CH₂OH HO OH HO HO OH OH OH f. sucrose CH₂OH OH OH HO OCH₂ OH a monosaccharide that gives a negative Benedict's Test. a ẞ-1,4'-glycoside a disaccharidearrow_forwardShow how each of the following transformations might be best accomplished. More than one step may required. Show all reagents and all intermediate structures. [4 only] CH3 A. CH CH2 C Br CH3 CH3 CH3CH2 C NH2 CH3 B OH any source of carbon N MIHarrow_forward
- Consider the reaction below to answer the following questions. 0 0 25 PS ES 1919sds-III msx H H + 5% NaOCH 3, CH3OHA O CH₂OH Jeiniog 2E1 gniwool of mor]. Ignibuloni 9vil 19 A B 11 >buoqm gniwollol so dass 101 tomboy boo-11Coble or to r ton auch i viw ninlaxs, noitsausbroo 152 lobla ogsbau ton 250b br A. Which carbonyl compound functions as the electrophile in this reaction? B. Draw the structure of the enolate ion that is generated during the course of this reaction. C. This reaction is an example of: a. a mixed Claisen condensation. b. C. d. a Dieckman condensation. a Michael reaction. a mixed aldol reaction. HD HDarrow_forwardConsider the reaction below to answer the following questions: 847 Acetoacetic ester can be prepared by the Claisen self-condensation reaction of ethyl acetate. H₁C 0 H 0 IL 유 || OCH2CH3 1. NaOEt, EtOH C 2. H₂O H3C CH₂ Cold not tobizmo. S OCH2CH3 A. Write the complete stepwise mechanism for this reaction. Show all electron flow with arrows and draw all intermediate structures. B. Ethyl acetate can be prepared from ethanol as the only organic starting material. Show all reagents and structures for all intermediates in this preparation. C. Give the structures of the ester precursors for the following Claisen condensation product and formulate the reaction. ou OELarrow_forwardA. What is the correct structure for a-D-glucopyranose? CH₂OH a HO HO- OH b HO HO- OH HOH₂C OH OH OH CH₂OH HO C. HO HO- OH OH CH₂OH OH OH B. Draw structures for the products you would expect to obtain from reaction of B-D-galactopyranose with each of the following reagents. Be sure to include all relevant stereochemistry. [FOUR only] A. CH, Ag₂O B. warm dilute HNO3 C. (CH3CO)20, pryridine D. NaBH in H₂O E. CH₂OH, HCI F. Br₂, H₂O HO CH₂OH HO- OH OH B-D-galactopyranosearrow_forward
- Give the major organic product(s) for each of the following reactions or reaction sequences. CH₂CN 5% NaOEt, EIOH سجد سی . بلی H 1. NaOCH, CH,OH CH3 OCH3 2 H₂O*arrow_forwardDraw the structures for each of the intermediates in the boxes provided for the synthesis below. 004 HNO F HO CHCO) D Dydre R.SO. 1.1 NO fe HO H.SO. 2. CC1 NOH HO MCL HNO, H.50.arrow_forward. Each of the following compounds can be prepared by a mixed aldol condensation reaction. Give the Cructures of the aldehyde and/or ketone precursors for each aldol product and formulate the reaction. 0 CH=CHCCH 3. Ph 1arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
