
EBK ORGANIC CHEMISTRY
6th Edition
ISBN: 9781260475685
Author: SMITH
Publisher: MCGRAW-HILL HIGHER EDUCATION
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2.5, Problem 17P
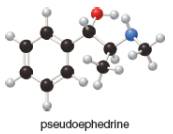
Which hydrogen in pseudoephedrine, the nasal decongestant in the
commercial medication Sudafed, is most acidic?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
PLEASE HELP! URGENT!
"Water gas" is an industrial fuel composed of a mixture of carbon monoxide and hydrogen gases. When this
fuel is burned, carbon dioxide and water result. From the information given below, write a balanced equation
and determine the enthalpy of this reaction:
CO(g) + O2(g) → CO₂(g) + 282.8 kJ
H2(g) + O2(g) → H₂O(g) + 241.8 kJ
MacBook Air
Page of 3
4. Calculate AG for the following reaction at 25°C. Will the reaction occur (be spontaneous)? How do you
know?
NH3(g) + HCl(g) → NH4Cl(s)
AH=-176.0 kJ
AS-284.8 J-K-1
Chapter 2 Solutions
EBK ORGANIC CHEMISTRY
Ch. 2.1 - a. Which compounds are Bronsted-Lowry acids:...Ch. 2.2 - a. Draw the conjugate acid of each base:...Ch. 2.2 - Label each statement as True or False.
a. is the...Ch. 2.2 - Decide which compound is the acid and which is the...Ch. 2.2 - Draw the products formed from the acid-base...Ch. 2.3 - Which compound in each pair is the stronger acid?...Ch. 2.3 - Use a calculator when necessary to answer the...Ch. 2.3 - Rank the conjugate bases of each of group of acids...Ch. 2.3 - Problem-2.10 Considers two acids: (formic acid,)...Ch. 2.3 - Prob. 11P
Ch. 2.4 - Draw the products of each reaction and determine...Ch. 2.4 - Prob. 13PCh. 2.5 - Without reference to a pKa table, decide which...Ch. 2.5 - Rank the labeled H atoms in the following compound...Ch. 2.5 - Which hydrogen in pseudoephedrine, the nasal...Ch. 2.5 - Which compound in each pair is the stronger acid?...Ch. 2.5 - Glycolic acid, HOCH2CO2H, is the simplest member...Ch. 2.5 - Explain the apparent paradox. HBr is a stronger...Ch. 2.5 - The CH bond in acetone, (CH3)2C=O, has a pKa of...Ch. 2.5 - Prob. 23PCh. 2.5 - For each pair of compounds: [1] Which indicated H...Ch. 2.5 - Rank the compounds in each group in order of...Ch. 2.5 - Prob. 26PCh. 2.5 - Prob. 27PCh. 2.6 - Prob. 28PCh. 2.7 - Problem 2.29
Compounds like amphetamine that...Ch. 2.8 - Problem 2.30 Which species are Lewis bases?
a. b....Ch. 2.8 - Which species are Lewis acids?
a. b. c. d.
Ch. 2.8 - For each reaction, label the Lewis acid and base....Ch. 2.8 - Prob. 33PCh. 2.8 - Prob. 34PCh. 2.8 - Label the Lewis acid and base. Use curved arrow...Ch. 2 - 2.36 Propranolol is an antihypertensive agent—that...Ch. 2 - 2.37 Amphetamine is a powerful stimulant of the...Ch. 2 - 2.38 What is the conjugate acid of each base?
a....Ch. 2 - 2.39 What is the conjugate base of each acid?
a....Ch. 2 - Draw the products of each proton transfer...Ch. 2 - Prob. 43PCh. 2 - What is Ka for each compound? Use a calculator...Ch. 2 - What is the pKa for each compound? a. b. c.Ch. 2 - Which of the following bases are strong enough to...Ch. 2 - Draw the products of each reaction. Use the pKa...Ch. 2 - a. What is the conjugate acid of A? b. What is the...Ch. 2 - Dimethyl ether (CH3OCH3) and ethanol (CH3CH2OH)...Ch. 2 - 2.59 Atenolol is a (beta) blocker, a drug used to...Ch. 2 - 2.60 Use the principles in Section 2.5 to label...Ch. 2 - 2.61 Label the three most acidic hydrogen atoms in...Ch. 2 - Prob. 66PCh. 2 - 2.63 Classify each compound as a Lewis base, a...Ch. 2 - 2.64 Classify each species as a Lewis acid, a...Ch. 2 - Label the Lewis acid and Lewis base in each...Ch. 2 - 2.66 Draw the products of each Lewis acid-base...Ch. 2 - Prob. 71PCh. 2 - 2.68 Answer the following questions about the four...Ch. 2 - Prob. 73PCh. 2 - 2.70 Hydroxide can react as a Brønsted-Lowry base...Ch. 2 - 2.71 Answer the following questions about esmolol,...Ch. 2 - Prob. 76PCh. 2 - Prob. 77PCh. 2 - Prob. 82P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- true or false The equilibrium constant for this reaction is 0.20. N2O4(g) ⇔ 2NO2(g) Based on the above, the equilibrium constant for the following reaction is 5. 4NO2(g) ⇔ 2N2O4(g)arrow_forwardtrue or false The equilibrium constant for this reaction is 0.20. N2O4(g) ⇔ 2NO2(g) Based on the above, the equilibrium constant for the following reaction is 0.4. 2N2O4(g) ⇔ 4NO2(g)arrow_forwardtrue or false Using the following equilibrium, if heat is added the equilibrium will shift toward the reactants. N2(g) + 3H2(g) ⇔ 2NH3(g) + heatarrow_forward
- True or False Using the following equilibrium, if heat is added the equilibrium will shift toward the products. N2O4(g) + heat ⇔ 2NO2(g)arrow_forwardtrue or false Using the following equilibrium, if solid carbon is added the equilibrium will shift toward the products. C(s) + CO2(g) ⇔ 2CO(g)arrow_forwardProvide the complete mechanism for the reaction below. You must include appropriate arrows,intermediates, and formal charges. Please also provide a reason to explain why the 1,4-adduct is preferred over the 1,3-adduct.arrow_forward
- Which of the following pairs are resonance structures of one another? I. III. || III IV + II. :0: n P !༠ IV. EN: Narrow_forwardPredict the major organic product(s) and byproducts (either organic or inorganic) for thefollowing reactions.arrow_forwardA 8.25 g sample of aluminum at 55°C released 2500 J of heat. The specific heat of aluminum is 0.900 J/g°C. The density of aluminum is 2.70 g/mL. Calculate the final temperature of the aluminum sample in °C.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY