
Interpretation:
The percentages of neutral and protonated forms present in a solution of 0.0010M pyrimidine at pH = 7.3 are to be calculated if the pKa of pyrimidinium ion is 1.3.
Concept introduction:
pH And pKa are related by Henderson-Hasselbalch equation as
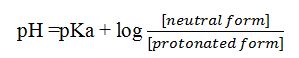
Knowing pH and pKa values, the ratio between the two forms and from which their percentages can be determined.
To calculate:
The percentages of neutral and protonated forms present in a solution of 0.0010M pyrimidine at pH = 7.3, if the pKa of pyrimidinium ion is 1.3.
Answer:
At pH = 7.3, almost 100% pyrimidine molecules exist in the neutral form.
Explanation:
Deprotonation of the ammonium ion of a base can be represented as,

The Henderson-Hasselbalch can be written as
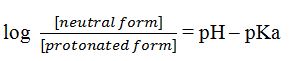
Substituting the values of pH and pKa, we get

Thus the concentration of neutral form is 106 times more than the protonated form. Hence almost 100% pyrimidine molecules exist in the neutral form.
Conclusion:
At pH = 7.3, almost 100% pyrimidine molecules exist in the neutral form.
Trending nowThis is a popular solution!

Chapter 24 Solutions
Study Guide with Student Solutions Manual for McMurry's Organic Chemistry, 9th
- Please see photoarrow_forward=Naming benzene derivatives Name these organic compounds: structure C1 CH3 name ☐ CH3 ப C1 × ☐arrow_forwardBlocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see image **NOTE: The compound on the left is the starting point, and the compound on the right is the final product. Please show the steps in between to get from start to final, please. These are not two different compounds that need to be worked.arrow_forward
- Nucleophilic Aromatic Substitution: What is the product of the reaction? What is the name of the intermediate complex? *See imagearrow_forwardPredict the final product. If 2 products are made, list which should be “major” and “minor” *see attachedarrow_forwardNucleophilic Aromatic Substitution: What is the product of the reaction? *see imagearrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax


