
ORGANIC CHEMISTRY-PRINT MULTI TERM
4th Edition
ISBN: 9781119832614
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Question
Chapter 24.2, Problem 4CC
(a)
Interpretation Introduction
Interpretation:
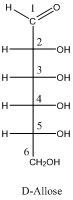
The Fischer projection of D-Allose and L-Allose are needed to be determined if the four chirality centers of D-Allose are in R-configuration.
Concept introduction:
- Fischer projection of carbohydrates are drawn by vertical line representing the cyclic carbon chain and the attached OH and H groups are placed in right or left side of each carbon atoms. The position of carbonyl group is on top of the line.
- R and S configuration of chiral carbons of carbohydrates are depending upon the location of –OH groups on the each carbon atom in Fischer projection.
- If the –OH group is in right side then, the configuration is R.
- If the –OH group is in left side then, the configuration is S.
- D and L configuration of a sugar are Enantiomers.
- Enantiomers are non-super imposable mirror images of each other.
To draw: the Fischer projection of D-Allose, if the four chirality centers of D-Allose are in R-configuration.
(b)
Interpretation Introduction
Interpretation:
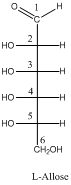
The Fischer projection of D-Allose and L-Allose are needed to be determined if the four chirality centers of D-Allose are in R-configuration.
Concept introduction:
- Fischer projection of carbohydrates are drawn by vertical line representing the cyclic carbon chain and the attached OH and H groups are placed in right or left side of each carbon atoms. The position of carbonyl group is on top of the line.
- R and S configuration of chiral carbons of carbohydrates are depending upon the location of –OH groups on the each carbon atom in Fischer projection.
- If the –OH group is in right side then, the configuration is R.
- If the –OH group is in left side then, the configuration is S.
- D and L configuration of a sugar are Enantiomers.
- Enantiomers are non-super imposable mirror images of each other.
To draw: the Fischer projection of L-Allose.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1/2
-
51%
+ »
GAY
Organic Reactions Assignment
/26
Write the type of reaction that is occurring on the line provided then complete the reaction. Only include the
major products and any byproducts (e.g. H₂O) but no minor products. Please use either full structural
diagrams or the combination method shown in the lesson. Skeletal/line diagrams will not be accepted.
H3C
1.
2.
CH3
A
Acid
OH
Type of Reaction:
NH
Type of Reaction:
+ H₂O
Catalyst
+ HBr
3.
Type of Reaction:
H3C
4.
Type Reaction:
5. H3C
CH2 + H2O
OH
+
[0]
CH3
Type of Reaction:
6. OH
CH3
HO
CH3 +
Type of Reaction:
7.
Type of Reaction:
+ [H]
humbnai
Concentration Terms[1].pdf ox + New
Home
Edit
Sign in
Comment
Convert
Page
Fill & Sign
Protect
Tools
Batch
+WPS A
Free Trial
Share
Inter Concreting Concentration forms.
Hydrogen peroxide is
a powerful oxidizing agent
wed in concentrated solution in rocket fuels and
in dilute solution as a
hair bleach. An aqueous
sulation of H2O2 is 30% by mass and has
density of #liligime calculat the
Ⓒmolality
⑥mole fraction of
molarity.
20
9.
B. A sample of Commercial Concentrated hydrochloric
ET
If a reaction occurs, what would be the major products? Please include a detailed explanation as well as a drawing showing how the reaction occurs and what the final product is.
Chapter 24 Solutions
ORGANIC CHEMISTRY-PRINT MULTI TERM
Ch. 24.2 - Prob. 1CCCh. 24.2 - Prob. 2CCCh. 24.2 - Prob. 3CCCh. 24.2 - Prob. 4CCCh. 24.2 - Prob. 5CCCh. 24.2 - Prob. 6CCCh. 24.4 - Prob. 7CCCh. 24.4 - Prob. 8CCCh. 24.5 - Prob. 1LTSCh. 24.5 - Prob. 9PTS
Ch. 24.5 - Prob. 2LTSCh. 24.5 - Prob. 12PTSCh. 24.5 - Prob. 13PTSCh. 24.5 - Prob. 3LTSCh. 24.5 - Prob. 16PTSCh. 24.5 - Prob. 19CCCh. 24.5 - Prob. 20CCCh. 24.5 - Prob. 21CCCh. 24.5 - Prob. 22CCCh. 24.6 - Prob. 23CCCh. 24.6 - Prob. 24CCCh. 24.6 - Prob. 25CCCh. 24.6 - Prob. 26CCCh. 24.6 - Prob. 27CCCh. 24.6 - Prob. 28CCCh. 24.6 - Prob. 29CCCh. 24.6 - Prob. 30CCCh. 24.6 - Prob. 4LTSCh. 24.6 - Prob. 31PTSCh. 24.6 - Prob. 34CCCh. 24.6 - Prob. 35CCCh. 24.6 - Prob. 36CCCh. 24.6 - Prob. 37CCCh. 24.6 - Prob. 38CCCh. 24.7 - Prob. 5LTSCh. 24.7 - Prob. 39PTSCh. 24.7 - Prob. 41CCCh. 24 - Prob. 42PPCh. 24 - Prob. 43PPCh. 24 - Prob. 44PPCh. 24 - Prob. 45PPCh. 24 - Prob. 46PPCh. 24 - Prob. 47PPCh. 24 - Prob. 48PPCh. 24 - Prob. 49PPCh. 24 - Prob. 50PPCh. 24 - Prob. 51PPCh. 24 - Prob. 52PPCh. 24 - Prob. 53PPCh. 24 - Prob. 54PPCh. 24 - Prob. 55PPCh. 24 - Prob. 56PPCh. 24 - Prob. 57PPCh. 24 - Prob. 58PPCh. 24 - Prob. 59PPCh. 24 - Prob. 60PPCh. 24 - Prob. 61PPCh. 24 - Prob. 62PPCh. 24 - Prob. 63PPCh. 24 - Prob. 64PPCh. 24 - Prob. 65PPCh. 24 - Prob. 66PPCh. 24 - Prob. 67PPCh. 24 - Prob. 68PPCh. 24 - Prob. 69PPCh. 24 - Prob. 70PPCh. 24 - Prob. 71PPCh. 24 - Prob. 72PPCh. 24 - Prob. 73PPCh. 24 - Prob. 74PPCh. 24 - Prob. 75PPCh. 24 - Prob. 76PPCh. 24 - Prob. 77PPCh. 24 - Prob. 78PPCh. 24 - Prob. 79PPCh. 24 - Prob. 80PPCh. 24 - Prob. 86IPCh. 24 - Prob. 87IPCh. 24 - Prob. 88IPCh. 24 - Prob. 89IP
Knowledge Booster
Similar questions
- Would the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forward(a) Sketch the 'H NMR of the following chemical including the approximate chemical shifts, the multiplicity (splitting) of all signals and the integration (b) How many signals would you expect in the 13C NMR? CH3arrow_forwardDraw the Show the major and minor product(s) for the following reaction mechanisms for both reactions and show all resonance structures for any Explain why the major product is favoured? intermediates H-Brarrow_forward
- 3. Draw ALL THE POSSBILE PRODUCTS AND THE MECHANISMS WITH ALL RESONANCE STRUCTURES. Explain using the resonance structures why the major product(s) are formed over the minor product(s). H₂SO4, HONO CHarrow_forward7. Provide the product(s), starting material(s) and/or condition(s) required for the No mechanisms required. below reaction HO + H-I CI FO Br2, FeBr3 O I-Oarrow_forward6. Design the most efficient synthesis of the following product starting from phenot Provide the reaction conditions for each step (more than one step is required) and explain the selectivity of each reaction. NO MECHANISMS ARE REQUIRED. OH step(s) CIarrow_forward
- What is the skeletal structure of the product of the following organic reaction?arrow_forwardIf a reaction occurs, what would be the major products? Please include a detailed explanation as well as a drawing showing how the reaction occurs and what the final product is.arrow_forwardWhat is the major organic product of the following nucleophilic acyl substitution reaction of an acid chloride below?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY