
Concept explainers
Interpretation:
The amount of ATP which is generated per gram of glucose in comparison to the amount of ATP generated per gram of arachidic acid during catabolism should be determined along with whether the result supports the fact that lipids are more effective energy-storing molecules in comparison to carbohydrates.
Concept introduction:
Carbohydrates are considered to be less effective than lipids in terms of releasing per gram consumption. But still, food rich in carbohydrate contents are more preferred over fats containing food. Energy produced by food containing high content of fat is almost double the amount of energy produced by food containing high content of carbohydrates.
Answer to Problem 99CP
The amount of which is generated per gram of glucose = 0.18mole ATP.
The amount of ATP generated per gram of arachidic acid during catabolism is equal to 0.43 mole ATP.
The number of ATP produced by complete catabolism of fat (fatty acid) is much higher than that of glucose (carbohydrate). Also, the energy produced by fat (fatty acid) is almost double the amount of energy produced by glucose (carbohydrate) by the complete catabolism. These results support the fact that lipids are more effective energy-storing molecules in comparison to carbohydrates.
Explanation of Solution
Glucose (a carbohydrate) starts with the glycolysis pathway which converts glucose to pyruvate.The initiation of the glycolysis process requires energy in the form of ATP. Total
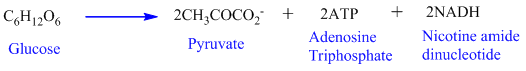
Transition reaction: On oxidation, pyruvate converted to acetyl CoA.
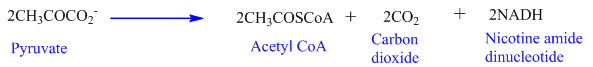
Citric acid Cycle:

From all the three reactions, total
Thus, the total
To calculate the amount of
As, fats (lipids) are metabolized within the body through
The first step is the investment of energy when
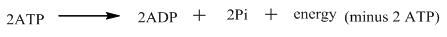
The number of acetyl
Each molecule
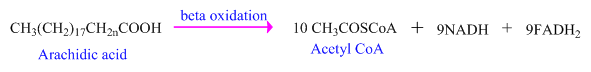
Acetyl
Every Acetyl
Thus, the total number of ATP generated during the cycle is
To calculate the amount of
From the calculation, it is clear that the number of ATP produced by complete catabolism of fat (fatty acid) is much higher than that of glucose (carbohydrate). Also, the energy produced by fat (fatty acid) is almost double the amount of energy produced by glucose (carbohydrate) by the complete catabolism. And these results supportthe factthat lipids (fats) store energy more effectively than carbohydrates.
Want to see more full solutions like this?
Chapter 24 Solutions
Loose Leaf for General, Organic and Biological Chemistry with Connect 2 Year Access Card
- identify the carbonyl compound that is incapable of forming an enolate ionarrow_forwardpredict the product formed by the reaction of one mole each of cyclohex-2-en-1-one and lithium diethylcuprate. Assume a hydrolysis step follows the additionarrow_forwardPlease handwriting for questions 1 and 3arrow_forward
- Is (CH3)3NHBr an acidic or basic salt? What happens when dissolved in aqueous solution? Doesn't it lose a Br-? Does it interact with the water? Please advise.arrow_forward© Macmilla Finish resonance structure 3 Select Draw Templates More C H N 0 H H S Erase Which structure is the most stable (lowest energy) resonance contributor? The structure with the positive charge on nitrogen and negative charges on oxygen and sulfur. All structures are equal in stability. The structure with the positive charge on nitrogen and negative charges on sulfur and carbon. The structure with the positive charge on nitrogen and negative charges on oxygen and carbon. Q2Qarrow_forwardThree pure compounds are formed when 1.00 g samples of element x combine with, respectively, 0.472 g, 0.630 g, and 0.789 g of element z. The first compound has the formula x2Z3. find the empricial formula of the other two compoundsarrow_forward
- Draw the product and the mechanism A. excess H*; 人 OH H*; B. C. D. excess OH ✓ OH H*; H₂O 1. LDA 2. H*arrow_forwardIn reactions whose kinetic equation is v = k[A]m, the rate coefficient k is always positive. Is this correct?arrow_forwardIf the concentration of A decreases exponentially with time, what is the rate equation? (A). -d[A] (B). dt d[A] = k[A] e-kt dtarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





