
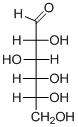
(a)
Interpretation:
The given aldohexoses should be identified.
Concept introduction:
Aldose: A monosaccharide in which carbon serves as a backbone and contains an
Ketose: A ketose is a simple sugar unit (monosaccharide) containing one
D and L enantiomers: L isomers have the hydroxyl group attached to the left side of the asymmetric carbon furthest from the carbonyl.
R isomers have the hydroxyl group attached to the right side of the asymmetric carbon furthest from the carbonyl.
D-Glyceraldehyde has the R configuration, while L-glyceraldehyde has the S configuration.
To find: Name of aldohexoses.
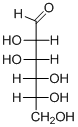
(b)
Interpretation:
The given aldohexoses should be identified.
Concept introduction:
Aldose: A monosaccharide in which carbon serves as a backbone and contains an aldehyde group at the beginning.
Ketose: A ketose is a simple sugar unit (monosaccharide) containing one ketone group per molecule.
D and L enantiomers: L isomers have the hydroxyl group attached to the left side of the asymmetric carbon furthest from the carbonyl.
R isomers have the hydroxyl group attached to the right side of the asymmetric carbon furthest from the carbonyl.
D-Glyceraldehyde has the R configuration, while L-glyceraldehyde has the S configuration.
To find: Name of aldohexoses.
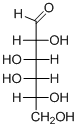
(c)
Interpretation:
The given aldohexoses should be identified.
Concept introduction:
Aldose: A monosaccharide in which carbon serves as a backbone and contains an aldehyde group at the beginning.
Ketose: A ketose is a simple sugar unit (monosaccharide) containing one ketone group per molecule.
D and L enantiomers: L isomers have the hydroxyl group attached to the left side of the asymmetric carbon furthest from the carbonyl.
R isomers have the hydroxyl group attached to the right side of the asymmetric carbon furthest from the carbonyl.
D-Glyceraldehyde has the R configuration, while L-glyceraldehyde has the S configuration.
To find: Name of aldohexoses.
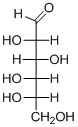
(d)
Interpretation:
The given aldohexoses should be identified.
Concept introduction:
Aldose: A monosaccharide in which carbon serves as a backbone and contains an aldehyde group at the beginning.
Ketose: A ketose is a simple sugar unit (monosaccharide) containing one ketone group per molecule.
D and L enantiomers: L isomers have the hydroxyl group attached to the left side of the asymmetric carbon furthest from the carbonyl.
R isomers have the hydroxyl group attached to the right side of the asymmetric carbon furthest from the carbonyl.
D-Glyceraldehyde has the R configuration, while L-glyceraldehyde has the S configuration.
To find: Name of aldohexoses.
Want to see the full answer?
Check out a sample textbook solution
Chapter 24 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
- K Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward7 Comment on the general features of the predicted (extremely simplified) ¹H- NMR spectrum of lycopene that is provided below. 00 6 57 PPM 3 2 1 0arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





