
Connect One Semester Access Card for General, Organic, & Biological Chemistry
4th Edition
ISBN: 9781260194654
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 24, Problem 29P
Interpretation Introduction
(a)
Interpretation:
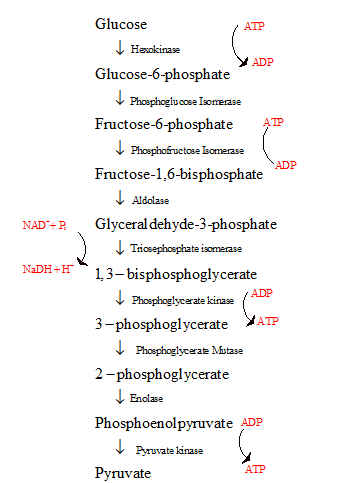
The energy investment phase and the energy generating phase of glycolysis with regard to the reactant that begins the phase and the final product formed are to be compared.
Concept Introduction:
- The glycolysis is the first step in the breakdown of glucose to pyruvate resulting in the release of energy in the form of ATP.
- The glycolysis cycle is represented as follows:
Interpretation Introduction
(b)
Interpretation:
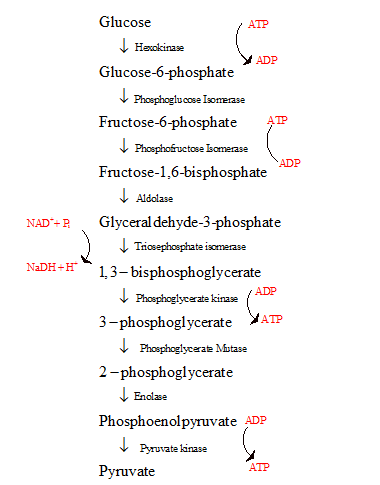
The energy investment phase and the energy generating phase of glycolysis with regard to the amount of ATP used or formed are to be compared.
Concept Introduction:
- The glycolysis is the first step in the breakdown of glucose to pyruvate resulting in the release of energy in the form of ATP.
- The glycolysis cycle is represented as follows:
Interpretation Introduction
(c)
Interpretation:
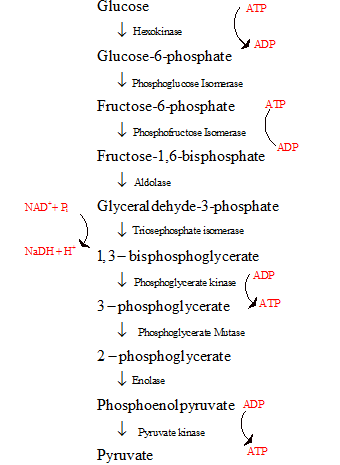
The energy investment phase and the energy generating phase of glycolysis with regard to the number of reduced co-enzymes used or formed are to be compared.
Concept Introduction:
- The glycolysis is the first step in the breakdown of glucose to pyruvate resulting in the release of energy in the form of ATP.
- The glycolysis cycle is represented as follows:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The Haber-Bosch process permits the direct conversion of molecular nitrogen to ammonia,
which can be used in large-scale fertilizer production. Given the balanced Haber-Bosch
reaction and using the bond energies in the table below, estimate the enthalpy change
associated with the reaction.
N2(g) + 3H2(g) → 2NH3(g)
Bond
N=N
N = N
Energy (kJ/mol)
941
418
N-N
H-H
N-H
163
435
388
Benzoic acid is used to determine the heat capacity of bomb calorimeters because it can be obtained in pure form and its energy of combustion is known very accurately (−26.43 kJ/g). Determine the heat capacity of a calorimeter that had a temperature increase of 9.199°C when 3.500 g of benzoic acid was used.
Given the standard enthalpies of formation for the following substances, determine the
reaction enthalpy for the following reaction.
2N2H4(g) + 2NO2(g) → 3N2(g) + 4H2O(g)
AHrxn
? kJ
Substance
AH in kJ/mol
N2H4(g)
+95.4
NO2(g)
+33.1
H2O(g)
-241.8
Chapter 24 Solutions
Connect One Semester Access Card for General, Organic, & Biological Chemistry
Ch. 24.2 - Analyze the following reaction by considering the...Ch. 24.2 - Prob. 24.2PPCh. 24.3 - Prob. 24.1PCh. 24.3 - Prob. 24.2PCh. 24.3 - Prob. 24.3PCh. 24.3 - Prob. 24.4PCh. 24.3 - Prob. 24.5PCh. 24.3 - Prob. 24.6PCh. 24.4 - Prob. 24.7PCh. 24.4 - Prob. 24.8P
Ch. 24.4 - Prob. 24.9PCh. 24.5 - Prob. 24.10PCh. 24.5 - Prob. 24.11PCh. 24.5 - Prob. 24.12PCh. 24.6 - Prob. 24.13PCh. 24.7 - Prob. 24.14PCh. 24.7 - Prob. 24.3PPCh. 24.7 - Prob. 24.15PCh. 24.7 - Prob. 24.16PCh. 24.7 - Use the number of molecules of ATP formed from the...Ch. 24.7 - Prob. 24.18PCh. 24.8 - Prob. 24.19PCh. 24.8 - Prob. 24.20PCh. 24.8 - Prob. 24.21PCh. 24.9 - Prob. 24.4PPCh. 24.9 - What products are formed when each amino acid is...Ch. 24.9 - Prob. 24.22PCh. 24 - Analyze each reaction by considering the...Ch. 24 - Analyze each reaction by considering the...Ch. 24 - Prob. 25PCh. 24 - Prob. 26PCh. 24 - Prob. 27PCh. 24 - Prob. 28PCh. 24 - Prob. 29PCh. 24 - Prob. 30PCh. 24 - Prob. 31PCh. 24 - Prob. 32PCh. 24 - Glucose is completely metabolized to six molecules...Ch. 24 - Why is glycolysis described as an anaerobic...Ch. 24 - Write the overall equation with key coenzymes for...Ch. 24 - Prob. 36PCh. 24 - Prob. 37PCh. 24 - Prob. 38PCh. 24 - Consider the aerobic and anaerobic avenues of...Ch. 24 - Prob. 40PCh. 24 - Prob. 41PCh. 24 - Prob. 42PCh. 24 - Prob. 43PCh. 24 - Prob. 44PCh. 24 - Prob. 45PCh. 24 - Prob. 46PCh. 24 - Prob. 47PCh. 24 - Prob. 48PCh. 24 - Prob. 49PCh. 24 - Prob. 50PCh. 24 - Prob. 51PCh. 24 - Prob. 52PCh. 24 - Prob. 53PCh. 24 - Prob. 54PCh. 24 - Prob. 55PCh. 24 - Prob. 56PCh. 24 - Prob. 57PCh. 24 - Prob. 58PCh. 24 - Prob. 59PCh. 24 - How much ATP is generated by the complete...Ch. 24 - Prob. 61PCh. 24 - Fill in the boxes with the number of moles of each...Ch. 24 - Prob. 63PCh. 24 - Prob. 64PCh. 24 - Prob. 65PCh. 24 - Prob. 66PCh. 24 - Prob. 67PCh. 24 - Prob. 68PCh. 24 - Prob. 69PCh. 24 - Prob. 70PCh. 24 - What is the difference between ketogenic and...Ch. 24 - Prob. 72PCh. 24 - Prob. 73PCh. 24 - Draw the structure of the keto acid formed by the...Ch. 24 - Draw the products formed in each transamination...Ch. 24 - Prob. 76PCh. 24 - Prob. 77PCh. 24 - Prob. 78PCh. 24 - Prob. 79PCh. 24 - Prob. 80PCh. 24 - What metabolic intermediate is formed from the...Ch. 24 - What metabolic intermediate is formed from the...Ch. 24 - Prob. 83PCh. 24 - Prob. 84PCh. 24 - Prob. 85PCh. 24 - Prob. 86PCh. 24 - Prob. 87PCh. 24 - What is the cause of the pain and cramping in a...Ch. 24 - Prob. 89PCh. 24 - Prob. 90PCh. 24 - Prob. 91PCh. 24 - Prob. 92PCh. 24 - Prob. 93PCh. 24 - Prob. 94PCh. 24 - What type of enzyme would catalyze the conversion...Ch. 24 - Prob. 96PCh. 24 - Prob. 97CPCh. 24 - Prob. 98CPCh. 24 - Prob. 99CPCh. 24 - Prob. 100CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If 7.3 kJ of energy are required to change the temperature of water from 5.0 to 70.0, what was the volume of water? (cs = 4.184 J/(g ⋅ ), d = 1.00 g/mL)arrow_forwardBALANCE CHEMICAL REACTIONarrow_forwardPredict the product(s) of the following reactions. If no reaction, write "NR". a) Cl₂ FeCl3 e) HNO3 H2SO4 b) NO2 CI. HNO3 f) Br Br2 OH H2SO4 HO3S. FeBr3 c) Cl2 g) FeCl3 F d) O₂N Br2 FeBr3 O₂N OH HNO3 CH3 H2SO4arrow_forward
- ulating the pH salt solution Calculate the pH at 25 °C of a 0.75M solution of anilinium chloride (C6H5NH3C1). Note that aniline (C6H5NH2) is a weak base with a pK of 4.87. Round your answer to 1 decimal place. pH = ☐ ☑ ⑤ ? olo 18 Ararrow_forwardI apologize, but the app is not allowing me to post the other 4 pictures of the thermodynamics chart. But I believe the values are universal. Please help!arrow_forwardCalculating the pH of a salt solution Calculate the pH at 25 °C of a 0.29M solution of potassium butanoate (KC3H,CO2). Note that butanoic acid (HC3H,CO2) is a weak acid with a pKa of 4.82. Round your answer to 1 decimal place. pH = -0 Х olo 18 Ararrow_forward
- : At a certain temperature, the equilibrium constant K for the following reaction is 1.58 × 10-12 N2(g) + O2(g) = 2 NO(g) Use this information to complete the following table. Suppose a 38. L reaction vessel is filled with 0.93 mol of N2 and 0.93 mol of O2. What can you say about the composition of the mixture in the vessel at equilibrium? There will be very little N2 and O2. There will be very little NO. What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 2 NO(g) N2(9)+02(9) What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 3 N2(9)+302(g) 6 NO(g) Neither of the above is true. K = ☐ K = ☐ ☐ X10 Х D ? 000 18 Ar Barrow_forwardwhen performing the reaction that involves 2 equivalents of 3-(diethylamino)-phenol and Phthalic anhydride with sulfuric acid and water react to form rhodamine b where the Phthalic anhydride cleaves in acid and how does Excessive Washing (w/ Base) & Subsequent Resonance Structure get affectedarrow_forward3. The strongest acid of the following compounds is ___.A. p-nitrophenol; B. m-nitrophenol; C. o-chlorophenol;D. p-methoxyphenol; E. o-methylphenol Please explain your steps and thought process. Thank you!arrow_forward
- Using the general properties of equilibrium constants At a certain temperature, the equilibrium constant K for the following reaction is 1.3 × 10 4: Cl2(g) + CHCl3(g) HCl(g) + CC₁(g) Use this information to complete the following table. Suppose a 16. L reaction vessel is filled with 1.6 mol of HCI and 1.6 mol of CCl4. What can you say about the composition of the mixture in the vessel at equilibrium? There will be very little Cl2 and CHCl3. ☐ x10 There will be very little HCI and CCl4. Neither of the above is true. What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. HCl(g)+CC14(g) 12 Cl2(9)+CHCl3(9) K = 0 ☐ What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 2 Cl₂(9)+2CHCl3(9) 2 HCl(9)+2CC₁₁(9) K = ✓ 00. 18 Ararrow_forward10. The most important reason why Br- is a better nucleophile than Cl-is ___. A. polarizability; B. size; C. solvation; D. basicity; E. polarity. Please include all steps. Thanks!arrow_forwardPredicting the qualitative acid-base properties of salts Consider the following data on some weak acids and weak bases: base acid Ка K₁₁ name formula name formula nitrous acid HNO2 4.5×10 4 pyridine CHEN 1.7 × 10 9 4 hydrofluoric acid HF 6.8 × 10 methylamine CH3NH2 | 4.4 × 10¯ Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. solution 0.1 M NaNO2 0.1 M KF pH choose one v choose one v 0.1 M C5H5NHBr 0.1 M CH3NH3CI choose one v ✓ choose one 1 (lowest) 2 ☑ 3 4 (highest) 000 18 Ararrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning