
Concept explainers
a)
Interpretation:
The structure of the
Concept introduction:
- The longest continuous ring of carbon atoms is identified. The prefix is added with ‘cyclo’.
- The substituent groups attached to the parent chain is identified. A substituent group contains group of atoms attached to the carbon atom of the chain.
- While numbering the longest chain, the substituent should get least possible number.
- Write the name of the compound; the parent name written as last part of the name. The name of the substituents is written as prefix and a hyphen separates the number that the substituents attached with carbon. More than one substituent should be written in alphabetical order.
a)
Explanation of Solution
Given name: cyclopentane
Predict the longest continuous chain of carbon atoms:
The parent name is CYCLOPENTANE represent the continuous ring of carbon atoms contains five carbons. The Suffix ‘ane’ represents the structure contains only single bonds.
Predict substituents and its location:
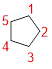
No prefix available before the parent name pentane, thus, the structure has no substituents located at any carbon atoms.
Therefore, the structure is

b)
Interpretation:
The structure of cis-2-butene has to be predicted.
Concept introduction:
IUPAC Nomenclature of
- The longest continuous chain of carbon atoms is identified.
- The substituent groups attached to the parent chain is identified. A substituent group contains group of atoms attached to the carbon atom of the chain.
- While numbering the longest chain, the substituent should get least possible number.
- Write the name of the compound; the parent name written as last part of the name. The name of the substituents is written as prefix and a hyphen separates the number that the substituents attached with carbon. More than one substituent should be written in alphabetical order.
Geometric isomers of Alkenes:
Cis-isomer: When two particular atoms (identical group of atoms) are adjacent (on same side) to each other, the alkene is known as cis-isomer.
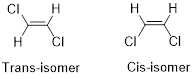
Trans-isomer: When two particular atoms (identical group of atoms) are opposite from each other, the alkene is known as trans-isomer.
b)
Explanation of Solution
Given name: cis-2-butene
Predict the longest continuous chain of carbon atoms:
The parent name is BUTENE represent the longest chain of carbon atoms contains four carbons. The Suffix ‘ene’ represents presence of double bond at C-2.
Predict substituents and its location:
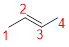
No prefix available before the parent name pentane, thus, the structure has no substituents located at any carbon atoms. The term ‘cis-’ indicates two hydrogen atoms are located adjacent to each other on same side.
Therefore, the structure is
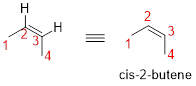
c)
Interpretation:
The structure of 2-hexanol has to be predicted.
Concept introduction:
IUPAC Nomenclature of alkanes:
- The longest continuous chain of carbon atoms is identified.
- The substituent groups attached to the parent chain is identified. A substituent group contains group of atoms attached to the carbon atom of the chain.
- While numbering the longest chain, the substituent should get least possible number.
- Write the name of the compound; the parent name written as last part of the name. The name of the substituents is written as prefix and a hyphen separates the number that the substituents attached with carbon. More than one substituent should be written in alphabetical order.
c)
Explanation of Solution
Given name: 2-hexanol
Predict the longest continuous chain of carbon atoms:
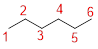
The parent name is HEXANOL represent the longest chain of carbon atoms contains six carbons. The Suffix ‘ol’ represents presence of hydroxy group –OH at C-2.
Predict substituents and its location:
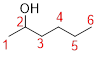
The prefix ‘2-‘represent the presence of hydroxy group at C-2.
Therefore, the structure is
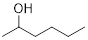
d)
Interpretation:
The structure of 1,4-dibromobenzene has to be predicted.
Concept introduction:
IUPAC Nomenclature of
- The parent compound of aromatic compound is Benzene.
- The substituent groups attached to the parent is identified. A substituent group contains group of atoms attached to the carbon atom of the aromatic ring.
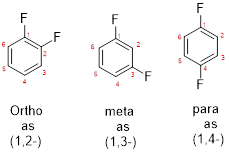
- While numbering the parent, the location of the second group relative to the first substituent uses prefixes like o-ortho (1,2-), m-meta (1,3-), and p-para (1,4) in disubstituted aromatic ring.
d)
Explanation of Solution
Given name: 1,4-dibromobenzene
Predict the parent structure:
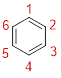
The parent name is BENZENE represent the continuous ring of carbon atoms contains six carbons with alternative double bonds.
Predict substituents and its location:
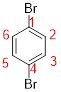
The prefix ‘1, 4-dibromo’ indicates the presence of two identical bromine atom located at C-1 and C-4.
Therefore, the structure is
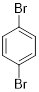
e)
Interpretation:
The structure of 2-butyne has to be predicted.
Concept introduction:
IUPAC Nomenclature of
- The longest continuous chain of carbon atoms is identified. The suffix is added replaced with ‘yne’.
- The substituent groups attached to the parent chain is identified. A substituent group contains group of atoms attached to the carbon atom of the chain.
- While numbering the longest chain, the substituent should get least possible number.
- Write the name of the compound; the parent name written as last part of the name. The name of the substituents is written as prefix and a hyphen separates the number that the substituents attached with carbon. More than one substituent should be written in alphabetical order.
e)
Explanation of Solution
Given name: 2-butyne
Predict the longest continuous chain of carbon atoms:

The parent name is BUTYNE represent the longest chain of carbon atoms contains four carbons. The Suffix ‘yne’ represents the structure contains only one triple bonds at C-2.
Predict substituents and its location:

No prefix available before the parent name pentane, thus, the structure has no substituents located at any carbon atoms.
Therefore, the structure is

Want to see more full solutions like this?
Chapter 24 Solutions
AVC LOOSELEAF CHEMISTRY W/CONNECT 2 SEM
- Show how to convert ethyl benzene to (a) 2,5-dichlorobenzoic acid and (b) 2,4-dichlorobenzoic acid.arrow_forwardno aiarrow_forwardPolymers may be composed of thousands of monomers. Draw three repeat units (trimer) of the polymer formed in this reaction. Assume there are hydrogen atoms there are hydrogen atoms on the two ends of the trimer. Ignore inorganic byproducts.arrow_forward
- 8:44 PM Sun Apr 13 Earn Freecash.com O Measurement and Matter =1 Setting up a unit conversion 110 Eddie says... ✰ www-awu.aleks.com A student sets up the following equation to convert a measurement. (The ? stands for a number the student is going to calculate.) Fill in the missing part of this equation. Note: your answer should be in the form of one or more fractions multiplied together. (- 4 J kJ -7.0 × 10 ☐ = ? mmol.°C mol °C x10 μ Explanation Check □·□ torox.io Grey Hill LLC. All Rightsarrow_forwardPolymers may be composed of thousands of monomers. Draw three repeat units (trimer) of the polymer formed in this reaction. Assume there are hydrogen atoms there are hydrogen atoms on the two ends of the trimer. Ignore inorganic byproducts please.arrow_forwardi need help with the folarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





