
(a)
Interpretation:
The organic product of the given reaction is to be drawn.
Concept introduction:
Multiple bonds in
Answer to Problem 24.58P
The structure of the reactant in the given reaction is
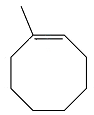
Explanation of Solution
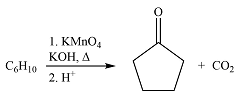
The given reaction is
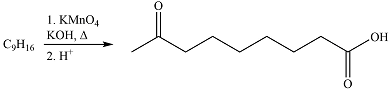
Hot, concentrated permanganate (
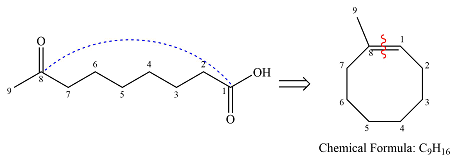
Therefore, the structure of the reactant must be

The structure of the reactant was determined based on retrosynthetic analysis, the undoing of oxidative cleavage of a double bond.
(b)
Interpretation:
The organic product of the given reaction is to be drawn.
Concept introduction:
Multiple bonds in alkenes and alkynes are cleaved when treated with hot, concentrated permanganate in a basic solution in an oxidative cleavage reaction. Both carbons of the alkyne are oxidized in the process to carboxyl groups. The end carbon of a terminal alkyne is oxidized completely to carbon dioxide. The final functional group that is formed depends on the reaction conditions. The permanganate ion is added across the double bond in a way similar to the Diels-Alder reaction, a
Answer to Problem 24.58P
The structure of the reactant in the given reaction is
Explanation of Solution
The given reaction is
Hot, concentrated permanganate (
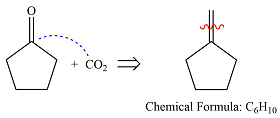
Therefore, the structure of the reactant must be

The structure of the reactant was determined based on retrosynthetic analysis, the undoing of oxidative cleavage of a double bond.
(c)
Interpretation:
The organic product of the given reaction is to be drawn.
Concept introduction:
Multiple bonds in alkenes are cleaved when treated with ozone in a reaction called ozonolysis. Both carbons of the alkene are oxidized in the process to carbonyl groups. Ozone adds across the double bond in a
Answer to Problem 24.58P
The structure of the reactant in the given reaction is
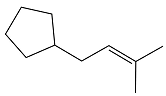
Explanation of Solution
The given reaction is
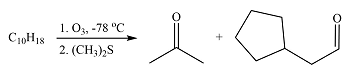
Ozonolysis will cleave the double bond in the reactant, oxidizing the two carbons to carbonyl groups. Therefore, the two carbonyl carbons in the products must have been joined by a double bond in the reactant.
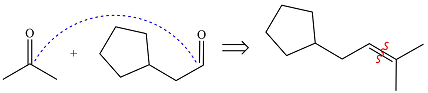
Therefore, the structure of the reactant must be

The structure of the reactant was determined based on retrosynthetic analysis, the undoing of oxidative cleavage of a double bond.
(d)
Interpretation:
The organic product of the given reaction is to be drawn.
Concept introduction:
Multiple bonds in alkenes are cleaved when treated with ozone in a reaction called ozonolysis. Both carbons of the alkene are oxidized in the process to carbonyl groups. Ozone adds across the double bond in a
Answer to Problem 24.58P
The structures of the reactant in the given reaction is
![]()
Explanation of Solution
The given reaction is
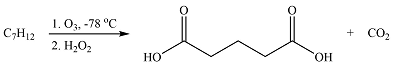
Ozonolysis will cleave a double bond in the reactant to oxidize the two carbons to carbonyl groups. If the product is an aldehyde, it is oxidized to the carboxylic acid group by
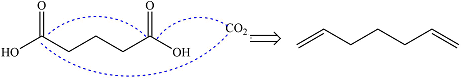
Therefore, the structure of the reactant must be
![]()
The structure of the reactant was determined based on retrosynthetic analysis, the undoing of oxidative cleavage of a double bond.
Want to see more full solutions like this?
Chapter 24 Solutions
EBK ORGANIC CHEMISTRY: PRINCIPLES AND M
- Nonearrow_forward29. Use frontier orbital analysis (HOMO-LUMO interactions) to decide whether the following dimerization is 1) thermally allowed or forbidden and 2) photochemically allowed or forbidden. +arrow_forward30.0 mL of 0.10 mol/L iron sulfate and 20.0 mL of 0.05 mol/L of silver nitrate solutions are mixed together. Justify if any precipitate would formarrow_forward
- Does the carbonyl group first react with the ethylene glycol, in an intermolecular reaction, or with the end alcohol, in an intramolecular reaction, to form a hemiacetal? Why does it react with the alcohol it does first rather than the other one? Please do not use an AI answer.arrow_forwardThe number of noncyclic isomers that have the composition C4H8Owith the O as part of an OH group, counting a pair of stereoisomers as1, is A. 8; B. 6; C. 9; D. 5; E. None of the other answers is correct.arrow_forwardNonearrow_forward
- The number of carbon skeletons that have 8 carbons, one of which istertiary is A. 7; B. More than 7; C. 6; D. 5; E. 4arrow_forwardThe azide ion is N3^-. In addition to the ionic charge, it’s three mostimportant contributing structures also have formal charges. The totalnumber of π bonds in these three contributing structures isA. 6; B. 12; C. 3; D. 9; E. None of the other answers is correct.arrow_forwardThe sum of the numerals in the name of the compoundis A. None of the other answers is correct.; B. 11;C. 6; D. 8; E. 5.arrow_forward
- A compound has a six carbon ring with three double bonds. Attachedto the ring is a three carbon chain with a triple bond and a two carbonchain with two bromines attached. The number of hydrogens in a molecule of this compound is A. 10; B. 12; C. 14; D. 13; E. None of the other answers is correct.arrow_forwardCan you help me? I can't seem to understand the handwriting for the five problems, and I want to be able to solve them and practice. If you'd like to give me steps, please do so to make it easier understand.arrow_forwardThe number of 2sp3 hybrid orbitals in the moleculeis A. 12; B. 8; C. 3; D. 11; E. None of the other answers is correct.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





