
Concept explainers
Fill in the lettered reagents needed for each reaction.
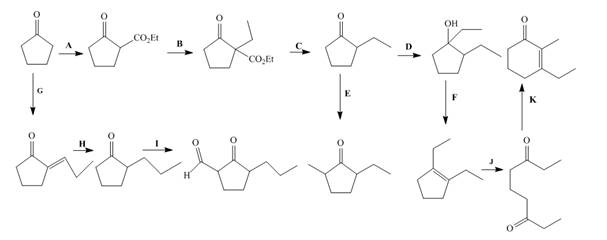
Interpretation: The lettered reagents that are needed for the given reactions are to be shown.
Concept Introduction: In crossed claisen condensation reaction, the base abstracts the acidic proton from
Answer to Problem 24.48P
The lettered reagents that are needed for the given reactions are shown below.
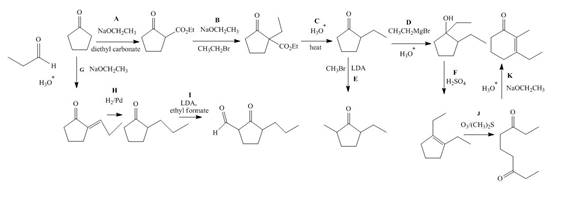
Explanation of Solution
The lettered reagent A is shown as,
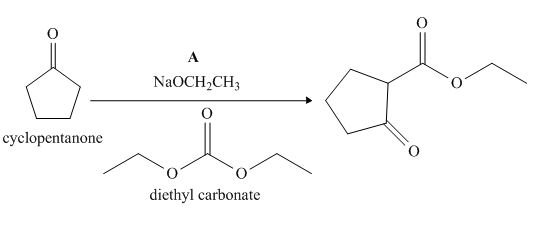
Figure 1
The given compound, cyclopentanone is treated with the base, sodium ethoxide that results in the formation of an enolate ion. Then, the enolate ion reacts with the compound, diethyl carbonate to form the desired compound,
The lettered reagent B is shown as,
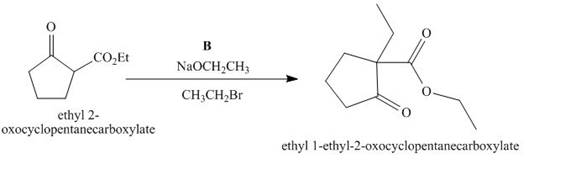
Figure 2
The compound,
The lettered reagent C is shown as,
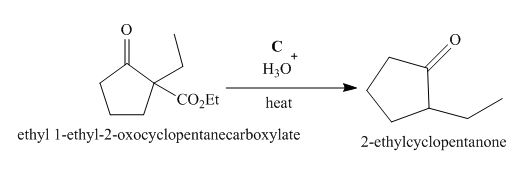
Figure 3
The compound,
The lettered reagent D is shown as,
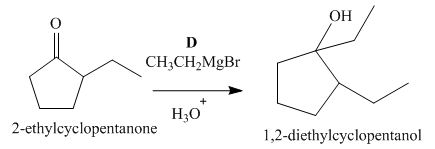
Figure 4
The compound,
The lettered reagent E is shown as,
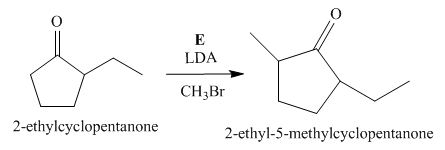
Figure 5
The compound,
The lettered reagent F is shown as,
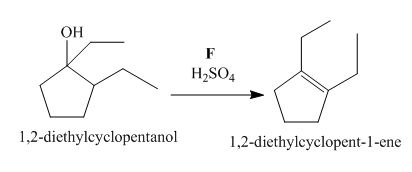
Figure 6
The compound,
The lettered reagent G is shown as,
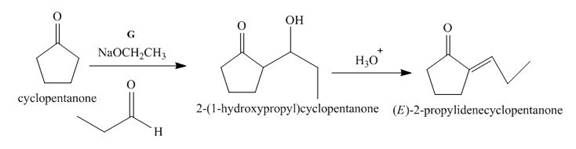
Figure 7
The given compound, cyclopentanone is treated with the base, sodium ethoxide that results in the formation of an enolate ion. Then, the enolate ion reacts with the compound, propanal to form the compound,
The lettered reagent H is shown as,
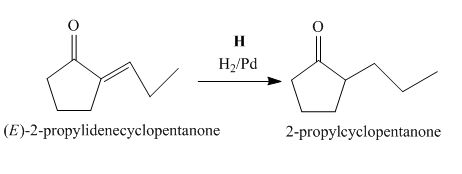
Figure 8
The compound,
The lettered reagent I is shown as,
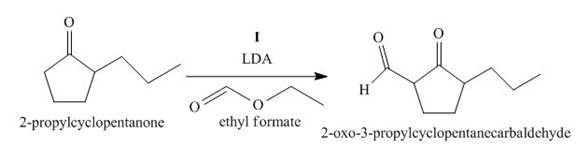
Figure 9
The compound,
The lettered reagent J is shown as,
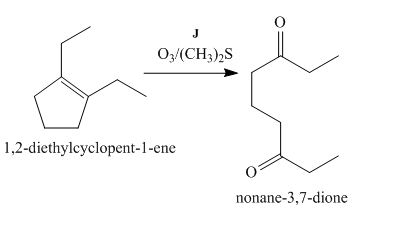
Figure 10
The compound,
The lettered reagent K is shown as,
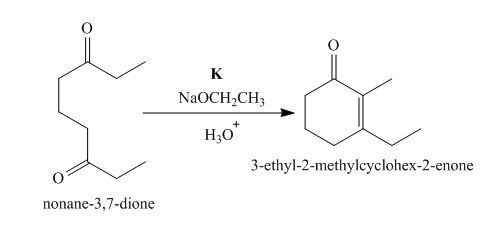
Figure 11
The intramolecular aldol reaction takes place in the compound,
The complete filled reagents that are needed for the given reactions are shown as,
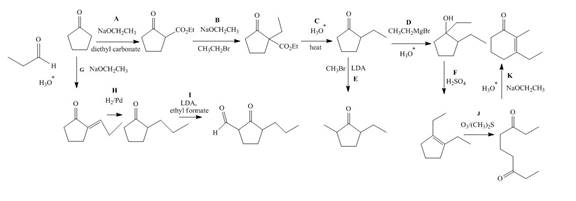
Figure 12
The lettered reagents that are needed for the given reactions are shown in Figure 12.
Want to see more full solutions like this?
Chapter 24 Solutions
Organic Chemistry
- Correctly name this compound using the IUPAC naming system by sorting the components into the correct order. Br IN Ν Harrow_forwardHow is the radical intermediate for this structure formed? Can you please draw arrows from the first radical to the resonance form that would result in this product? I'm lost.arrow_forwardPart VI. (a) calculate the λ max of the compound using woodward - Fieser rules. (b) what types of electronic transitions are present in the compound? (c) what are the prominent peaks in the IR spectrum of the compound?arrow_forward
- Don't used Ai solutionarrow_forwardPlease correct answer and don't used hand raitingarrow_forward↑ 0 Quiz List - RCC430M_RU05 X Aktiv Learning App × Qdraw resonance structure ×Q draw resonance structure xb My Questions | bartleby ×+ https://app.aktiv.com Draw a resonance structure of pyrrole that has the same number of pi bonds as the original structure. Include all lone pairs in your structure. + N H a 5 19°F Cloudy Q Search Problem 12 of 15 Atoms, Bonds and Rings Charges and Lone Pairs myhp हजु Undo Reset Remove Done Submit Drag To Pan 2:15 PM 1/25/2025arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

