
(a)
Interpretation:
The curved arrows for a concerted mechanism for each given reaction are to be drawn.
Concept introduction:
Curved arrows are drawn cyclically to account for the movement of electrons.
Curved arrows in the concerted mechanism are drawn from the number of electrons move in the reaction,
Answer to Problem 24.37P
The curved arrows for a concerted mechanism for each given reaction are:
(i)
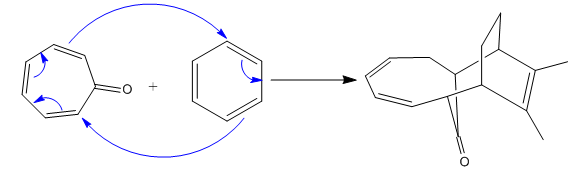
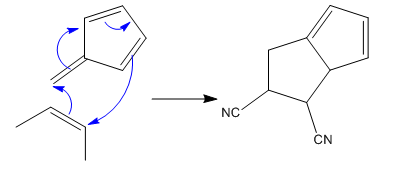
(ii)
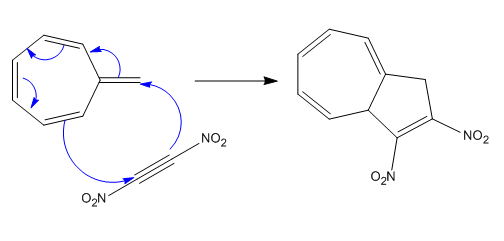
(iii)
Explanation of Solution
(i)
The given reaction is:
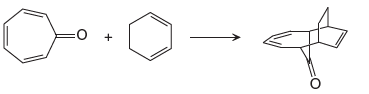
In the above reaction, one reactant has

(ii)
The given reaction is:
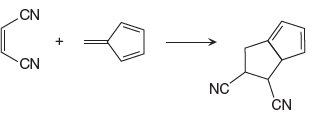
In the above reaction, one reactant has

(iii)
The given reaction is:
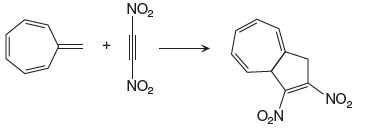
In the above reaction, one reactant has

The curved arrows for a concerted mechanism for the given reaction are drawn using the total number of
(b)
Interpretation:
The transition state formed in each given reaction is to be drawn.
Concept introduction:
Cycloaddition reactions proceeds through the cyclic transition state which represents the breaking and making of bonds.
Answer to Problem 24.37P
The transition state formed in the each given reaction is:
(i)
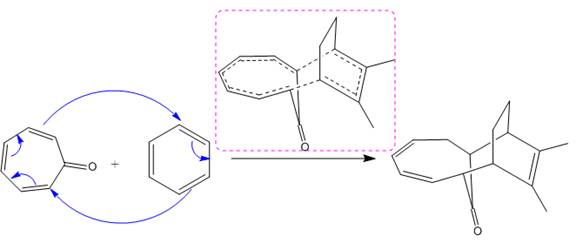
(ii)
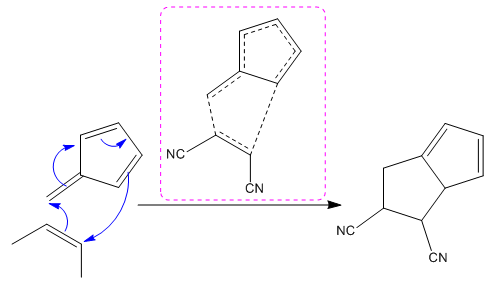
(iii)
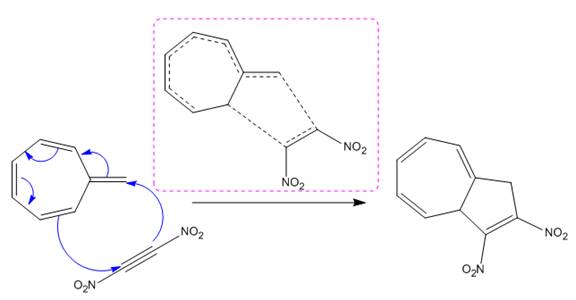
Explanation of Solution
(i)
The given reaction is:

The above cycloaddition reaction proceeds through a cyclic transition state, represents the breaking and making of bond by dash bond.
The transition state had shown the total number of
The transition state formed in the above reaction is marked in the box shown below:
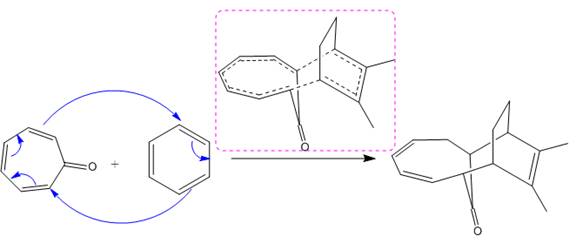
(ii)
The given reaction is:

The above cycloaddition reaction proceeds through a cyclic transition state that represents the breaking and making of the bond by dash bond.
The transition state had shown the total number of
The transition state formed in the above reaction is marked in the box shown below:

(iii)
The given reaction is:

The above cycloaddition reaction proceeds through a cyclic transition state that represents the breaking and making of the bond by dash bond.
The transition state had shown the total number of
The transition state formed in the above reaction is marked in the box show below:
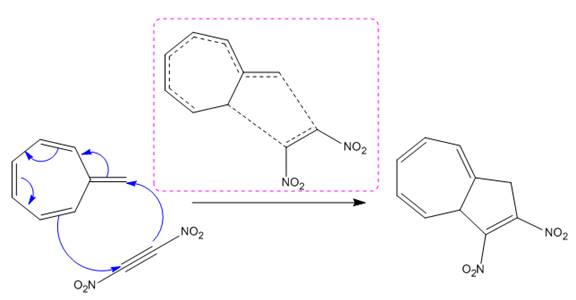
The transition state formed in the each given reaction is drawn using the curved arrows direction with number of
(c)
Interpretation:
The nature of transition state either
Concept introduction:
The transition state aromatic if the number of electrons in that cyclic
Answer to Problem 24.37P
(i) The transition state is aromatic.
(ii) The transition state is antiaromatic
(iii) The transition state is aromatic.
Explanation of Solution
(i)
The concerted mechanism for the given reaction is:
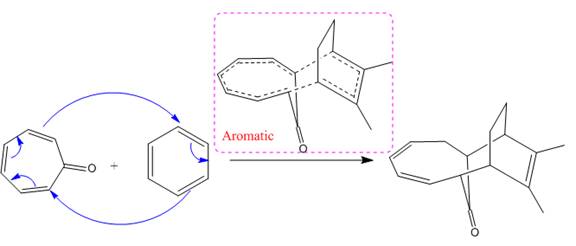
In the above reaction,
(ii)
The concerted mechanism for the given reaction is:
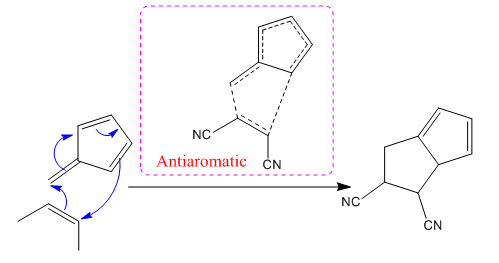
In the above reaction,
(iii)
The concerted mechanism for the given reaction is:
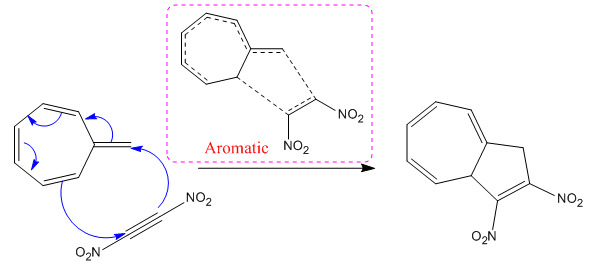
In the above reaction,
The nature of transition state either aromatic or antiaromatic for each reaction is identified from the number of
(d)
Interpretation:
Based on the answer to part (c), whether the reaction is allowed or forbidden under normal conditions is to be identified.
Concept introduction:
The reaction proceeds through aromatic transition state is allowed whereas if the transition state is antiaromatic the reaction is forbidden.
Answer to Problem 24.37P
(i) The given reaction is allowed under normal conditions.
(ii) The given reaction is forbidden under normal conditions.
(iii) The given reaction is allowed under normal conditions.
Explanation of Solution
(i)
The given reaction proceeds through the aromatic transition state, i.e., the number of moving
(ii)
The given reaction proceeds through the antiaromatic transition state, i.e., the number of moving
(iii)
The given reaction proceeds through the aromatic transition state, i.e., the number of moving
Whether the reaction is allowed or forbidden under normal conditions is identified from the nature of the transition state form in the respective reaction.
Want to see more full solutions like this?
Chapter 24 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- ↓ ina xSign x Sign X labs X Intro X Cop Xa chat X My Cx Grac X Laur x Laur xash learning.com/ihub/assessment/f188d950-dd73-11e0-9572-0800200c9a66/d591b3f2-d5f7-4983-843c-0d00c1c0340b/f2b47861-07c4-4d1b-a1ee-e7db2 +949 pts /3400 K Question 16 of 34 > © Macmillan Learning Draw the major E2 reaction product formed when cis-1-chloro-2-ethylcyclohexane (shown) reacts with hydroxide ion in DMSO. H CH2CH3 H H HO- H H H Cl DMSO H H C Select Draw Templates More C H 0 2 Erasearrow_forwardA common buffer for stabilizing antibodies is 100 mM Histidine at pH 7.0. Describe the preparation of this buffer beginning with L-Histidine monohydrochloride monohydrate and 1 M NaOH. Be certain to show the buffering reaction that includes the conjugate acid and base.arrow_forwardFina x | Sign X Sign X lab: X Intro X Cop) X a chat x My x Grad xLaur x Laur x a sheg X S Shoj XS SHE X acmillanlearning.com/ihub/assessment/f188d950-dd73-11e0-9572-0800200c9a66/d591b3f2-d5f7-4983-843c-0d00c1c0340b/f2b47861-07c4-4d1b-a1ee-e7db27d6b4ee?actualCourseld=d591b3f2- 5 © Macmillan Learning Organic Chemistry Maxwell presented by Macmillan Learning For the dehydrohalogenation (E2) reaction shown, draw the Zaitsev product, showing the stereochemistry clearly. H H KOH Br EtOH Heat Select Draw Templates More Erase // C H Q Search hp Q2 Q Δ קו Resouarrow_forward
- Is the structural form shown possible given the pKa constraints of the side chains?arrow_forwardon x Fina X Sign X Sign x lab X Intro X Cop X chat X My x Grac x Laur x Laur x ashes x S Shox S SHE x a eve.macmillanlearning.com/ihub/assessment/f188d950-dd73-11e0-9572-0800200c9a66/d591b3f2-d5f7-4983-843c-0d00c1c0340b/f2b47861-07c4-4d1b-a1ee-e7db27d6b4ee?actualCourseld=d591b3f2-c stions estion. ct each urces. +95 Macmillan Learning Draw the product formed by the reaction of potassium t-butoxide with (15,25)-1-bromo-2-methyl-1-phenylbutane (shown). Clearly show the stereochemistry of the product. H BH (CH3)3CO-K+ +100 H3CW (CH3)3COH +85 H3CH2C +95 ossible ↓ Q Search Select Draw Templates More C H 0 bp A Erase 2Q 112 Resouarrow_forwardIdentify the structure of the PTH derivative generated after two rounds of Edman degradation.arrow_forward
- Use the data below from an electron impact mass spectrum of a pure compound to deduce its structure. Draw your structure in the drawing window. Data selected from the NIST WebBook, https://webbook.nist.gov/chemistry/ m/z Relative intensity 31 0.5 30 26 29 22 28 100 27 33 26 23 15 4 • You do not have to consider stereochemistry. You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. 妊 n ? Previous Nextarrow_forwardfor this question. Write the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M+ = 98.1106. Exact Masses of the Most Abundant Isotope of Selected Elements Isotope Natural abundance (%) Exact mass 1H 99.985 1.008 12C 98.90 12.000 14N 99.63 14.003 160 99.76 15.995 Molecular formula (In the order CHNO, with no subscripts)arrow_forwardPLEASE READ!!! I DONT WANT EXAMPLES, I DONT WANT WORDS OR PARAGRAPHS!!! PLEASE I UNDERSTAND THE BASICS BUT THIS IS AN EXCEPTION THAT EVEN THE INTERNET CANT HELP!!!! THIS IS THE THIRD TIME I'VE SENT THOSE QUESTIONS SO PLEASE DONT RESEND THE SAME STUFF, ITS NOT HELPING ME!!! I ALSO ALREADY TRIED TO DRAW THE MECHANISM MYSELF, SO IF ITS RIGHT PLEASE TELL ME OR TELL ME WHAT I HAVE TO CHANGE!!! First image: I have to SHOW (DRAWING) the mechanism (with arows and structures of molecules) NOT WORDS PLEASE! of the reaction at the bottom. Also I have to show by mecanism why the reaction wouldn't work if the alcohol was primary Second image: I have to show the mechanism (IMAGE) (with arrows and structures of the molecules) NOT WORDS PLEASE !! for the reaction on the left, where the alcohol A is added fast in one portion HOMEWORK, NOT EXAM!! ALL DETAILS ARE IN THE IMAGES PLEASE LOOK AT THE IMAGES, DONT LOOK AT THE AI GENERATED TEXT!!!arrow_forward
- Write the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M+ = 85.0899. Exact Masses of the Most Abundant Isotope of Selected Elements Isotope Natural abundance (%) Exact mass 1H 99.985 1.008 12C 98.90 12.000 14N 99.63 14.003 160 99.76 15.995 Molecular formula (In the order CHNO, with no subscripts)arrow_forwardUse the data below from an electron impact mass spectrum of a pure compound to deduce its structure. Draw your structure in the drawing window. Data selected from the NIST WebBook, https://webbook.nist.gov/chemistry/ m/z Relative intensity 59 3.0 58 64 43 100 15 23 • You do not have to consider stereochemistry. •You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. + n[] 85 // ? CH4 Previous Nextarrow_forwardWrite the molecular formula for a compound with the possible elements C, H, N and O that exhibits a molecular ion at M* = 128.0632. Exact Masses of the Most Abundant Isotope of Selected Elements Isotope Natural abundance (%) Exact mass 1H 99.985 12C 98.90 14N 99.63 160 99.76 Molecular formula 1.008 12.000 14.003 15.995 (In the order CHNO, with no subscripts)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





