
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 24, Problem 21P
Interpretation Introduction
Interpretation:
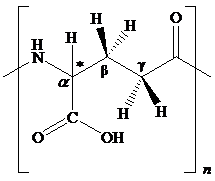
The structural feature of polyglutamic acid and the chemical change for given transformation are to be explained.
Concept introduction:
The
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Identify and provide an explanation of the differences between homogeneous and heterogeneous sampling in the context of sampling methods.
Г
C-RSA CHROMATOPAC CH=1
DATA 1: @CHRM1.C00
ATTEN=10 SPEED= 10.0
0.0 b.092
0.797
1.088
1.813
C-RSA CHROMATOPAC CH=1 Report No. =13
** CALCULATION REPORT **
DATA=1: @CHRM1.000 11/03/05 08:09:52
CH PKNO
TIME
1
2
0.797
3
1.088
4
1.813
AREA
1508566
4625442
2180060
HEIGHT
207739
701206 V
287554 V
MK IDNO
CONC
NAME
18.1447
55.6339
26.2213
TOTAL
8314067 1196500
100
C-R8A CHROMATOPAC CH=1
DATA 1: @CHRM1.C00
ATTEN=10 SPEED= 10.0
0. 0
087
337.
0.841
1.150
C-R8A CHROMATOPAC CH=1 Report No. =14
DATA=1: @CHRM1.000 11/03/05 08:12:40
** CALCULATION REPORT **
CH PKNO
TIME
AREA
1
3 0.841
1099933
41.15
4039778
HEIGHT MK IDNO
170372
649997¯¯¯
CONC
NAME
21.4007
78.5993
TOTAL
5139711 820369
100
3
C-R8A CHROMATOPAC
CH=1
DATA 1: @CHRM1.C00
ATTEN=10 SPEED= 10.0
0.100
0:652
5.856
3
1.165
C-RSA CHROMATOPAC CH-1 Report No. =15
DATA=1: @CHRM1.000 11/03/05 08:15:26
** CALCULATION REPORT **
CH PKNO TIME
AREA
HEIGHT MK IDNO CONC
NAME
1 3 3 0.856
4
1.165
TOTAL
1253386
4838738
175481
708024 V
20.5739
79.4261
6092124…
Draw the product of the reaction shown below. Ignore small byproducts that would evaporate please.
Chapter 24 Solutions
Organic Chemistry
Ch. 24 - Prob. 1PPCh. 24 - Practice Problem 24.2 The guanidino group NHNHCNH2...Ch. 24 - Prob. 3PPCh. 24 - Prob. 4PPCh. 24 - Prob. 5PPCh. 24 - Prob. 6PPCh. 24 - Prob. 7PPCh. 24 - Practice Problem 24.8
Glutathione is a tripeptide...Ch. 24 - Prob. 9PPCh. 24 - Prob. 10PP
Ch. 24 - Prob. 11PPCh. 24 - Practice Problem 24.12 Show all steps in the...Ch. 24 - Practice Problem 24.13 The synthesis of a...Ch. 24 - Practice Problem 24.14
The terminal carboxyl...Ch. 24 - Prob. 15PPCh. 24 - Prob. 16PPCh. 24 - (a) Which amino acids in Table 24.1 have more than...Ch. 24 - Prob. 18PCh. 24 - 24.19 (a) On the basis of the following sequence...Ch. 24 - Prob. 20PCh. 24 - Prob. 21PCh. 24 - Prob. 22PCh. 24 - Prob. 23PCh. 24 - Prob. 24PCh. 24 - The enzyme lysozyme and its mechanism are...Ch. 24 - Prob. 2LGPCh. 24 - 24.1 Write the structural formula of the principal...Ch. 24 - Prob. 2Q
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Relative Abundance 20- Problems 501 (b) The infrared spectrum has a medium-intensity peak at about 1650 cm. There is also a C-H out-of-plane bending peak near 880 cm. 100- 80- 56 41 69 M(84) LL 15 20 25 30 35 55 60 65 70 75 80 85 90 m/zarrow_forwardPolyethylene furanoate is a polymer made from plant-based sources; it is used for packaging. Identify the monomer(s) used in the production of this polymer using a condensation process.arrow_forwardPhenol is the starting material for the synthesis of 2,3,4,5,6-pentachlorophenol, known al-ternatively as pentachlorophenol, or more simply as penta. At one time, penta was widely used as a wood preservative for decks, siding, and outdoor wood furniture. Draw the structural formula for pentachlorophenol and describe its synthesis from phenol.arrow_forward
- 12 Mass Spectrometry (d) This unknown contains oxygen, but it does not show any significant infrared absorption peaks above 3000 cm . 59 100- BO 40 Relative Abundance M(102) - 15 20 25 30 35 40 45 50 5 60 65 70 75 80 85 90 95 100 105 mizarrow_forwardDraw a Haworth projection of a common cyclic form of this monosaccharide: H HO H HO H HO H H -OH CH2OH Click and drag to start drawing a structure. Х : Darrow_forward: Draw the structure of valylasparagine, a dipeptide made from valine and asparagine, as it would appear at physiological pH. Click and drag to start drawing a structure. P Darrow_forward
- Draw the Haworth projection of α-L-mannose. You will find helpful information in the ALEKS Data resource. Click and drag to start drawing a structure. : ཊི Х Darrow_forwardDraw the structure of serine at pH 6.8. Click and drag to start drawing a structure. : d كarrow_forwardTake a look at this molecule, and then answer the questions in the table below it. CH2OH H H H OH OH OH CH2OH H H H H OH H H OH H OH Is this a reducing sugar? yes α β ロ→ロ no ☑ yes Does this molecule contain a glycosidic bond? If you said this molecule does contain a glycosidic bond, write the symbol describing it. O no 0+0 If you said this molecule does contain a glycosidic bond, write the common names (including anomer and enantiomer labels) of the molecules that would be released if that bond were hydrolyzed. If there's more than one molecule, separate each name with a comma. ☐arrow_forward
- Answer the questions in the table below about this molecule: H₂N-CH₂ -C—NH–CH–C—NH–CH—COO- CH3 CH CH3 What kind of molecule is this? 0= CH2 C If you said the molecule is a peptide, write a description of it using 3-letter codes separated ☐ by dashes. polysaccharide peptide amino acid phospolipid none of the above Хarrow_forwardDraw a Haworth projection of a common cyclic form of this monosaccharide: CH₂OH C=O HO H H -OH H OH CH₂OH Click and drag to start drawing a structure. : ☐ Х S '☐arrow_forwardNucleophilic Aromatic Substitution 22.30 Predict all possible products formed from the following nucleophilic substitution reactions. (a) (b) 9 1. NaOH 2. HCI, H₂O CI NH₁(!) +NaNH, -33°C 1. NaOH 2. HCl, H₂Oarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning