
Organic Chemistry, Books a la Carte Edition (8th Edition)
8th Edition
ISBN: 9780134074580
Author: Bruice, Paula Yurkanis
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 23.5, Problem 13P
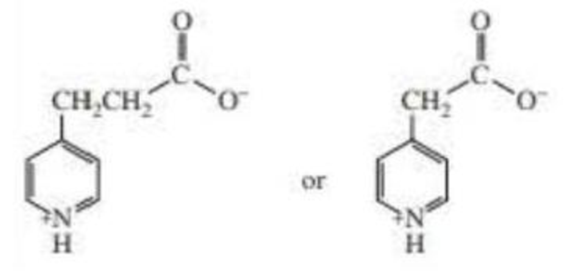
Which compound is more easily decarboxylated?
Expert Solution & Answer
Learn your wayIncludes step-by-step video

schedule03:18
Students have asked these similar questions
How are the molecules or ions in each pair related? Classify them as resonance structures, isomers, or neither.
Which of the given resonance structures (A, B, or C) contributes most to the resonance hybrid? Which contributes least? Provide steps and explanation
PLEASE HELP NOW URGENT!
Chapter 23 Solutions
Organic Chemistry, Books a la Carte Edition (8th Edition)
Ch. 23.1 - Prob. 2PCh. 23.1 - Prob. 3PCh. 23.2 - How many conjugated double bonds are there in a....Ch. 23.2 - Instead of adding to the 4a position and...Ch. 23.2 - Prob. 7PCh. 23.3 - Prob. 8PCh. 23.3 - Acetolactate synthase is another TPP-requiring...Ch. 23.3 - Acetolactate synthase transfers the acyl group of...Ch. 23.3 - Prob. 12PCh. 23.5 - Which compound is more easily decarboxylated?
Ch. 23.5 - Prob. 14PCh. 23.5 - Explain why the ability of PLP to catalyze an...Ch. 23.5 - Explain why the ability of PLP to catalyze an...Ch. 23.5 - The enzyme that catalyzes the C C bond cleavage...Ch. 23.5 - Propose a mechanism for the ,-elimination reaction...Ch. 23.6 - Ethanolamine ammonia lyase, a coenzyme...Ch. 23.6 - Prob. 20PCh. 23.7 - How do the structure of tetrahydrofolate and...Ch. 23.7 - What is the source of the methyl group in...Ch. 23.8 - Thiols such as ethanethiol and propanethiol can be...Ch. 23 - How does the metal ion in carboxypeptidase A...Ch. 23 - Prob. 24PCh. 23 - Prob. 25PCh. 23 - For each of the following reactions, name both the...Ch. 23 - Prob. 27PCh. 23 - When transaminated, the three branched-chain amino...Ch. 23 - What acyl groups have we seen transferred by...Ch. 23 - Propose a mechanism for the following reaction:Ch. 23 - Draw the products of the following reaction, where...Ch. 23 - When UMP is dissolved in T2O, exchange of T for H...Ch. 23 - Dehydratase is a PLP-requiring enzyme that...Ch. 23 - In addition to the reaction mentioned in Section...Ch. 23 - PLP can catalyze both ,-elimination reactions...Ch. 23 - The glycine cleavage system is a group of four...Ch. 23 - Prob. 37PCh. 23 - FADH2 reduces , -unsaturated thioesters to...
Additional Science Textbook Solutions
Find more solutions based on key concepts
An obese 55-year-old woman consults her physician about minor chest pains during exercise. Explain the physicia...
Biology: Life on Earth with Physiology (11th Edition)
How can the freezing of water crack boulders?
Campbell Biology in Focus (2nd Edition)
Pigeons may exhibit a checkered or plain color pattern. In a series of controlled matings, the following data w...
Concepts of Genetics (12th Edition)
[14.110] The following mechanism has been proposed for the gas-phase reaction of chloroform (CHCI3) and chlorin...
Chemistry: The Central Science (14th Edition)
15. In the Olympic shotput event, an athlete throws the shot with an initial speed of 12.0 m/s at a 40.0° angle...
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
16. Explain some of the reasons why the human species has been able to expand in number and distribution to a g...
Campbell Biology: Concepts & Connections (9th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- HELP NOW PLEASE URGENTarrow_forwardHow do I solve this Alkyne synthesis homework problem for my Organic Chemistry II class? I have to provide both the intermediate products and the reagents used.arrow_forwardSubstance X is known to exist at 1 atm in the solid, liquid, or vapor phase, depending on the temperature. Additionally, the values of these other properties of X have been determined: melting point enthalpy of fusion 90. °C 8.00 kJ/mol boiling point 130. °C enthalpy of vaporization 44.00 kJ/mol density 2.80 g/cm³ (solid) 36. J.K mol (solid) 2.50 g/mL (liquid) heat capacity 32. J.Kmol (liquid) 48. J.Kmol (vapor) You may also assume X behaves as an ideal gas in the vapor phase. Ex Suppose a small sample of X at 50 °C is put into an evacuated flask and heated at a constant rate until 15.0 kJ/mol of heat has been added to the sample. Graph the temperature of the sample that would be observed during this experiment. o0o 150- 140 130- 120- 110- 100- G Ar ?arrow_forward
- Mechanism. Provide the mechanism for the reaction below. You must include all arrows, intermediates, and formal charges. If drawing a Sigma complex, draw all major resonance forms. The ChemDraw template of this document is available on Carmen. Br FeBr3 Brarrow_forwardCheck the box under each compound that exists as a pair of mirror-image twins. If none of them do, check the none of the above box under the table. CH3 OH CH3 CH2 -CH-CH3 CH3 OH OH CH-CH2-CH- -CH3 CH3 CH3 OH OH CH3 C -CH2- C. -CH3 CH3- -CH2- -CH-CH2-OH OH CH3 none of the above كarrow_forwardWrite the systematic name of each organic molecule: structure Η OH OH OH OH H namearrow_forward
- Draw the skeletal ("line") structure of a secondary alcohol with 5 carbon atoms, 1 oxygen atom, at least one ring, and no double or triple bonds. Click and drag to start drawing a structure. : ☐ ☑ ⑤arrow_forwardName these organic compounds: structure name CH₁₂ CH3 - C CH - CH2 || CH3- - CH₂ CH₂ | - - CH3 CH3 2-methyl-2-butene ☐ 3-methyl-1-butyne - CH3 CH. - C=CHarrow_forwardHow many different molecules are drawn below?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License