
Concept explainers
(a)
Interpretation:
The
Concept introduction:
Organic compounds are named systematically by using IUPAC rules.
Name of the organic compounds are given according to the number of carbon present in the molecule for example
A molecule having one carbon atom, the molecule name will start with meth etc.…

If any halogens are present in the molecule, the name of the halogens as follows.

Naming the substituted
- (1) Name the parent alkane (long alkyl chain)
- (2) Number the carbon
- (3) Name and number the substituent
If the molecules have the multiple substituents, the compound named as di, tri, tetra, Penta, etc..
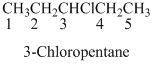
If the molecules having functional group, the name of the compound is given below. Numbering should be starts from the functional group of the given molecule.
The given compound is an alcohol
Example is given below
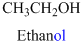

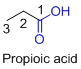
The given compound is an acid (
The amides are derivatives of acids and it is named as the ending of alkane with amide.
For example
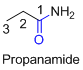
If the molecule is ester,
Esters end with “ate”
Example
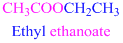
The given compound is an
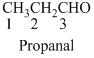
The given compound is a
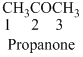
The given compound is an
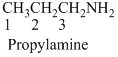
(b)
Interpretation:
The functional group should be identified for the given molecule.
Concept introduction:
Organic compounds are named systematically by using IUPAC rules.
Name of the organic compounds are given according to the number of carbon present in the molecule for example
A molecule having one carbon atom, the molecule name will start with meth etc.…

If any halogens are present in the molecule, the name of the halogens as follows.

Naming the substituted alkane:
- (4) Name the parent alkane (long alkyl chain)
- (5) Number the carbon
- (6) Name and number the substituent
If the molecules have the multiple substituents, the compound named as di, tri, tetra, Penta, etc..
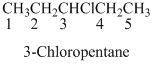
If the molecules having functional group, the name of the compound is given below. Numbering should be starts from the functional group of the given molecule.
The given compound is an alcohol
Example is given below
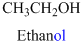

The given compound is an acid (

The amides are derivatives of acids and it is named as the ending of alkane with amide.
For example
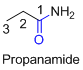
If the molecule is ester,
Esters end with “ate”
Example
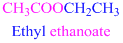
The given compound is an aldehyde (
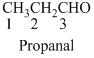
The given compound is a ketone (
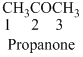
The given compound is an amine (
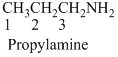
(c)
Interpretation:
The functional group should be identified for the given molecule.
Concept introduction:
Organic compounds are named systematically by using IUPAC rules.
Name of the organic compounds are given according to the number of carbon present in the molecule for example
A molecule having one carbon atom, the molecule name will start with meth etc.…

If any halogens are present in the molecule, the name of the halogens as follows.

Naming the substituted alkane:
- (7) Name the parent alkane (long alkyl chain)
- (8) Number the carbon
- (9) Name and number the substituent
If the molecules have the multiple substituents, the compound named as di, tri, tetra, Penta, etc..
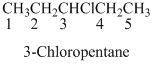
If the molecules having functional group, the name of the compound is given below. Numbering should be starts from the functional group of the given molecule.
The given compound is an alcohol
Example is given below
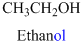

The given compound is an acid (

The amides are derivatives of acids and it is named as the ending of alkane with amide.
For example
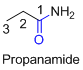
If the molecule is ester,
Esters end with “ate”
Example
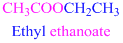
The given compound is an aldehyde (
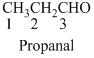
The given compound is a ketone (
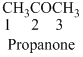
The given compound is an amine (
Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
Chemistry: Atoms First V1
- 2. Predict the product(s) that forms and explain why it forms. Assume that any necessary catalytic acid is present. .OH HO H₂N OHarrow_forwardconsider the rate of the reaction below to be r. Whats the rate after each reaction? Br + NaCN CN + NaBr a. Double the concentration of alkyl bromide b. Halve the concentration of the electrophile & triple concentration of cyanide c. Halve the concentration of alkyl chloridearrow_forwardPredict the organic reactant that is involved in the reaction below, and draw the skeletal ("line") structures of the missing organic reactant. Please include all steps & drawings & explanations.arrow_forward
- What are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardWhat is the organic molecule X of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forwardWhat are is the organic molecule X and product Y of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forward
- At 300 K, in the decomposition reaction of a reactant R into products, several measurements of the concentration of R over time have been made (see table). Without using graphs, calculate the order of the reaction. t/s [R]/(mol L-1) 0 0,5 171 0,16 720 0,05 1400 0,027arrow_forwardPredict the organic products that form in the reaction below, and draw the skeletal ("line") structures of the missing organic products. Please include all steps & drawings & explanations.arrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forward
- What are the products of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forwardWhat would happen if you added the HCI to the Grignard reagent before adding benzophenone? Draw a reaction mechanism to support your answer.arrow_forwardAt 300 K, in the decomposition reaction of a reactant R into products, several measurements of the concentration of R over time have been made (see table). Calculate the order of the reaction. t/s [R]/ (mol L-1) 0 0,5 171 0,16 720 0,05 1400 0,027arrow_forward
 Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





