
Concept explainers
(a)
Interpretation:
The preparation for given compound should be identified using benzene and any other reagents.
Concept Introduction:
Heck reaction: It describes the coupling reaction between aryl, vinyl or benzyl halide and
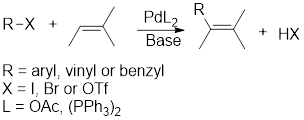
(b)
Interpretation:
The preparation for given compound should be identified using benzene and any other reagents.
Concept Introduction:
Negishi reaction: It describes how the product obtained when
(c)
Interpretation:
The preparation for given compound should be identified using benzene and any other reagents.
Concept Introduction:
Heck reaction: It describes the coupling reaction between aryl, vinyl or benzyl halide and alkene in presence of
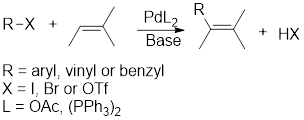
Simmons–Smith reaction: The reaction involves formation of cyclopropane using reagents namely
(d)
Interpretation:
The preparation for given compound should be identified using benzene and any other reagents.
Concept Introduction:
Heck reaction: It describes the coupling reaction between aryl, vinyl or benzyl halide and alkene in presence of
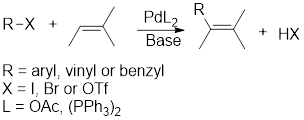
Simmons–Smith reaction: The reaction involves formation of cyclopropane using reagents namely
Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
EBK ORGANIC CHEMISTRY AS A SECOND LANGU
- Show how you would accomplish the following transformations. More than one step may be required. ow all reagents and all intermediate structures [one ONLY] A. H Br H CH3 NHz CH3 CH3 B. CH3CH2C-Br CH3CH2C-CN CH3 CH3.arrow_forwardShow how you would accomplish the following transformations. More than one step may be required. now all reagents and all intermediate structures [one ONLY] A. H Br H CH3 NHz CH3 CH3 B. CH3CH2C-Br CH3 CH3CH2C-CN CH3arrow_forwardCan I please get help with this?arrow_forward
- C. I, II, III Consider the reaction sequence below to answer the following questions: 0 0 1. NaOEt, EtOH ΕΙΟ OEt 2 Compound X CO₂Et NaOEt, EtOH CO₂Et Br Compound Y A Compound Z A. Compound X, diethyl propanedioate, is more commonly known as a. ethyl acetoacetate acetoacetic ester b. C. oxalic ester d. malonic ester B. Write the complete stepwise mechanism for the conversion of Compound X into Compound Y. Show all electron flow with arrows and draw all intermediate structures.arrow_forwardDiethyl malonate can be prepared by the following reaction sequence. Draw the structures of each of the missing intermediates in the boxes provided EtO 0 H3C 11 C 1. Br₂ PBr OH 2 H₂O 010 0 CH3CH₂OH C CH2 OEt Ha CH3CH2OH на NaCN H₂SO4 NC H₂O, heat CH2 OCH2CH3arrow_forwardShow how you would accomplish each of the following transformations. More than one step may be quired. Show all reagents and all intermediate structures. [three only] A. 0 CH3 B. C. D. H 0 0 OCH 3 CH₂CO₂CH2CH3 H3C ➤ HN C NO₂ Clarrow_forward
- Choose the BEST reagent for carrying out each of the following conversions. A. CO₂CH3 CO₂CH3 0 CO₂H a. LiAlH4, ether C. CrO3, pyridine B. 0 H a. C. NaBH4, ethanol NaOH, H2O CO₂H OH HD b. NaBH4, ethanol d. H₂/Pd CH₂OH b. CH₂PPh3 d. All of the abovearrow_forwardWrite the complete stepwise mechanism for the acid-catalyzed hydrolysis of the following amide to yield mandelic acid. Show all electron flow with arrows and draw the structures of all intermediate species. OH H-OH₂ CnH2 :0: OH C OH + NH4 10: The purpose of the acid catalyst in the hydrolysis of an amide is: to enhance the electrophilicity of the amide carbonyl carbon a. to enhance the nucleophilicity of the water molecule b. C. to enhance the electrophilicity of the water molecule d. to shift the equilibrium of the reactionarrow_forward1.arrow_forward
- Can I please get help with this?arrow_forward. Provide IUPAC names for each of the following structures OR draw structures corresponding to each of the following names: [Three only]kk a. H₂N- 0 COCH2CH3 benzocaine b. What is the correct structure for phenylbenzoate? C a. 0 C-O O b. H3C-C-O 0 0 C-O-CH3 d. CH₂O C-CHZ c. Acetyl chloride d. 3,4,5-trimethoxybenzoyl chloridearrow_forward. Draw structures corresponding to each of the following names or Provide IUPAC names for each of the ollowing structures [for 4 ONLY]. A. 2-propylpentanoic acid. B. m-chlorobenzoic acid. C. 0 0 HOC(CH2) COH glutaricadd D. E. F. 0 OH HO OH HO INCO salicylicadd H3C CH3 C=C tgicadd H COOH CH₂C=N 4arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





