
EBK ORGANIC CHEMISTRY AS A SECOND LANGU
3rd Edition
ISBN: 9781118203774
Author: Klein
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Question
Chapter 23, Problem 33PP
Interpretation Introduction
Interpretation:
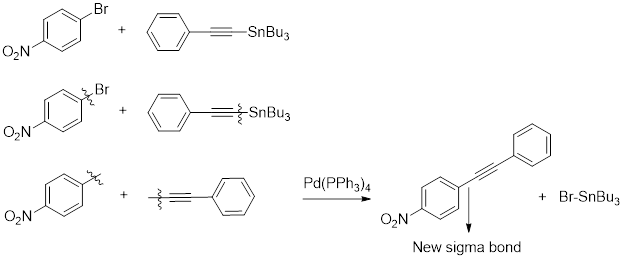
The coupling product obtained when given para–nitrobromobenzene treated with alkynylstannane in presence of
Concept Introduction:
Stille coupling: The reaction that involves coupling of an aryl, benzyl or vinyl halide or triflate with stannane in presence of
Expert Solution & Answer
Explanation of Solution
Analyzing both the given reactant clearly shows that the carbon atom present in
Want to see more full solutions like this?
Subscribe now to access step-by-step solutions to millions of textbook problems written by subject matter experts!
Students have asked these similar questions
>
You are trying to decide if there is a single reagent you can add that will make the following synthesis possible without any other
major side products:
1. ☑
CI
2. H3O+
O
Draw the missing reagent X you think will make this synthesis work in the drawing area below.
If there is no reagent that will make your desired product in good yield or without complications, just check the box under the
drawing area and leave it blank.
Click and drag to start drawing a
structure.
Explanation
Check
?
DO
18
Ar
B
© 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
Don't use ai to answer I will report you answer
Consider a solution of 0.00304 moles of 4-nitrobenzoic acid (pKa = 3.442) dissolved in 25 mL water and titrated with 0.0991 M NaOH. Calculate the pH at the equivalence point
Chapter 23 Solutions
EBK ORGANIC CHEMISTRY AS A SECOND LANGU
Ch. 23.1 - Identify which of the following reagents is...Ch. 23.2 - Prob. 2CCCh. 23.2 - Prob. 3CCCh. 23.2 - Prob. 4CCCh. 23.2 - Prob. 5CCCh. 23.2 - Prob. 6CCCh. 23.3 - Show how you would prepare 1-butylcyclopentene...Ch. 23.3 - Prob. 7PTSCh. 23.3 -
Using any two organohalides of your choice...Ch. 23.3 - Prob. 9ATS
Ch. 23.4 - Prob. 10CCCh. 23.4 - Prob. 11CCCh. 23.5 - Prob. 2LTSCh. 23.6 - Prob. 3LTSCh. 23.6 - Prob. 16PTSCh. 23.6 - Prob. 17PTSCh. 23.6 - Prob. 18ATSCh. 23.6 - Prob. 19ATSCh. 23.7 - Prob. 4LTSCh. 23.7 - Prob. 20PTSCh. 23.7 - Prob. 21PTSCh. 23.7 - Prob. 22ATSCh. 23.7 - Prob. 23ATSCh. 23.8 - Prob. 5LTSCh. 23.8 - Prob. 24PTSCh. 23.8 - Prob. 25ATSCh. 23.8 - Prob. 26ATSCh. 23.9 - Prob. 27CCCh. 23.9 - Prob. 28CCCh. 23.9 - Prob. 29CCCh. 23.9 - Prob. 6LTSCh. 23.9 - Prob. 30PTSCh. 23.9 - Prob. 31PTSCh. 23.9 - Prob. 32ATSCh. 23 - Prob. 33PPCh. 23 - Prob. 34PPCh. 23 - Prob. 35PPCh. 23 - Prob. 36PPCh. 23 - Prob. 37PPCh. 23 - Prob. 38PPCh. 23 - Prob. 39PPCh. 23 - Prob. 40PPCh. 23 - Prob. 41PPCh. 23 - Prob. 42PPCh. 23 - Prob. 43PPCh. 23 - Prob. 44PPCh. 23 - Prob. 45PPCh. 23 - Prob. 46PPCh. 23 - Using 1-pentene as your only source of carbon...Ch. 23 - Prob. 48PPCh. 23 - Prob. 49PPCh. 23 - Prob. 50PPCh. 23 - Prob. 51PPCh. 23 - Prob. 52PPCh. 23 - Prob. 53PPCh. 23 - Prob. 54PPCh. 23 - Prob. 55PPCh. 23 - Prob. 56PPCh. 23 - Prob. 57PPCh. 23 - Prob. 58PPCh. 23 - Prob. 59IPCh. 23 - Prob. 60IPCh. 23 - Prob. 61IPCh. 23 - Prob. 62IPCh. 23 - Prob. 64IPCh. 23 - Prob. 66IPCh. 23 - Prob. 68IPCh. 23 - Prob. 69IPCh. 23 - Prob. 70IPCh. 23 - Prob. 71CPCh. 23 - Prob. 72CPCh. 23 - Prob. 73CPCh. 23 - Prob. 74CPCh. 23 - Prob. 75CPCh. 23 - Prob. 76CP
Knowledge Booster
Similar questions
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY