
Concept explainers
Interpretation:
Two compounds are to be identified on the basis of their chemical formulas, and IR and
Concept introduction:
Peaks in an IR spectrum help in determining
In 13C NMR spectroscopy, we get information about the different types of carbon present in the given molecular formula.
13C NMR spectroscopy can even differentiate between primary, secondary, tertiary, and quaternary carbons.
If the compound only contains C, H, and O, the index of hydrogen deficiency is calculated as
If a compound contains C, H, O, and a halogen, the halogen can be treated as if it were a hydrogen for the purpose of calculating index of hydrogen deficiency.
Answer to Problem 35P
Solution:
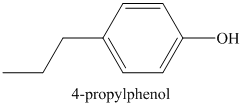
a) The structure of the compound
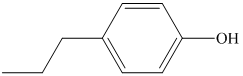
b) The structure of the compound
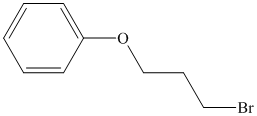
Explanation of Solution
The chemical formula of the compound is
The count of four indicates the possibility of a benzene ring. If a benzene ring is present, the oxygen atom would be singly bonded, either as a hydroxyl group or as an ether functional group.
The IR and 13C NMR spectra of the compound are as follows:
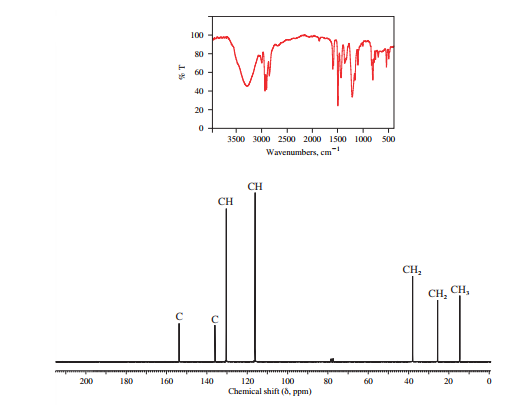
The presence of a broad peak around 3200
Chemical formula of the compound is
Therefore this compound also is likely to have a benzene ring.
The IR and 13C NMR spectra of the compound are as follows:
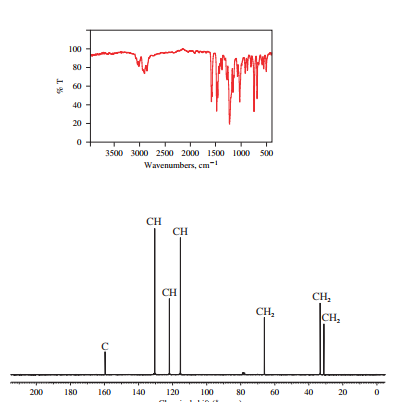
Two peaks in the IR spectrum, at about 700 and 750
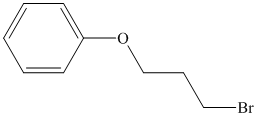
Therefore, the structure of the compound is
Want to see more full solutions like this?
Chapter 23 Solutions
ORGANIC CHEMISTRY (LL)-W/SOLN.>CUSTOM<
- State the name and condensed formula of isooxazole obtained by reacting acetylacetone and hydroxylamine.arrow_forwardState the name and condensed formula of the isothiazole obtained by reacting acetylacetone and thiosemicarbazide.arrow_forwardProvide the semi-developed formula of isooxazole obtained by reacting acetylacetone and hydroxylamine.arrow_forward
- Given a 1,3-dicarbonyl compound (R1-CO-CH2-CO-R2), indicate the formula of the compound obtaineda) if I add hydroxylamine (NH2OH) to give an isooxazole.b) if I add thiosemicarbazide (NH2-CO-NH-NH2) to give an isothiazole.arrow_forwardAn orange laser has a wavelength of 610 nm. What is the energy of this light?arrow_forwardThe molar absorptivity of a protein in water at 280 nm can be estimated within ~5-10% from its content of the amino acids tyrosine and tryptophan and from the number of disulfide linkages (R-S-S-R) between cysteine residues: Ε280 nm (M-1 cm-1) ≈ 5500 nTrp + 1490 nTyr + 125 nS-S where nTrp is the number of tryptophans, nTyr is the number of tyrosines, and nS-S is the number of disulfide linkages. The protein human serum transferrin has 678 amino acids including 8 tryptophans, 26 tyrosines, and 19 disulfide linkages. The molecular mass of the most dominant for is 79550. Predict the molar absorptivity of transferrin. Predict the absorbance of a solution that’s 1.000 g/L transferrin in a 1.000-cm-pathlength cuvet. Estimate the g/L of a transferrin solution with an absorbance of 1.50 at 280 nm.arrow_forward
- In GC, what order will the following molecules elute from the column? CH3OCH3, CH3CH2OH, C3H8, C4H10arrow_forwardBeer’s Law is A = εbc, where A is absorbance, ε is the molar absorptivity (which is specific to the compound and wavelength in the measurement), and c is concentration. The absorbance of a 2.31 × 10-5 M solution of a compound is 0.822 at a wavelength of 266 nm in a 1.00-cm cell. Calculate the molar absorptivity at 266 nm.arrow_forwardHow to calculate % of unknown solution using line of best fit y=0.1227x + 0.0292 (y=2.244)arrow_forward
- Given a 1,3-dicarbonyl compound, state the (condensed) formula of the compound obtaineda) if I add hydroxylamine (NH2OH) to give an isooxazole.b) if I add thiosemicarbazide (NH2-CO-NH-NH2) to give an isothiazole.arrow_forwardComplete the following acid-base reactions and predict the direction of equilibrium for each. Justify your prediction by citing pK values for the acid and conjugate acid in each equilibrium. (a) (b) NHs (c) O₂N NH NH OH H₁PO₁arrow_forward23.34 Show how to convert each starting material into isobutylamine in good yield. ཅ ནད ཀྱི (b) Br OEt (c) (d) (e) (f) Harrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

