
Student Study Guide and Solutions Manual for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
8th Edition
ISBN: 9781305864504
Author: Brent L. Iverson, Sheila Iverson
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 23, Problem 23.49P
Interpretation Introduction
Interpretation:
The synthesis has to be shown for the given compounds.
Concept introduction:
Nitration: The formation of nitro group in a
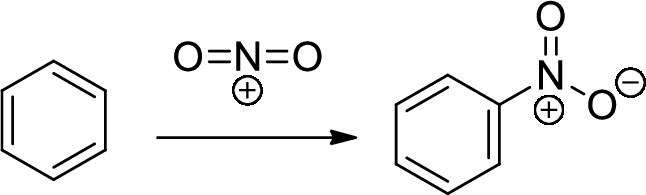
Reduction: nitro group undergoing reduction by using reducing agent like metals like Ni or Pt with hydrogen which provides
Reduction:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Lab Data
The distance entered is out of the expected range.
Check your calculations and conversion factors.
Verify your distance. Will the gas cloud be closer to the cotton ball with HCI or NH3?
Did you report your data to the correct number of significant figures?
- X
Experimental Set-up
HCI-NH3
NH3-HCI
Longer Tube
Time elapsed (min)
5 (exact)
5 (exact)
Distance between cotton balls (cm)
24.30
24.40
Distance to cloud (cm)
9.70
14.16
Distance traveled by HCI (cm)
9.70
9.80
Distance traveled by NH3 (cm)
14.60
14.50
Diffusion rate of HCI (cm/hr)
116
118
Diffusion rate of NH3 (cm/hr)
175.2
175.2
How to measure distance and calculate rate
For the titration of a divalent metal ion (M2+) with EDTA, the stoichiometry of the reaction is typically:
1:1 (one mole of EDTA per mole of metal ion)
2:1 (two moles of EDTA per mole of metal ion)
1:2 (one mole of EDTA per two moles of metal ion)
None of the above
Please help me solve this reaction.
Chapter 23 Solutions
Student Study Guide and Solutions Manual for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
Ch. 23.1 - Prob. 23.1PCh. 23.2 - Prob. 23.2PCh. 23.2 - Prob. 23.3PCh. 23.2 - Prob. 23.4PCh. 23.5 - Prob. 23.5PCh. 23.5 - Prob. AQCh. 23.5 - What is the hybridization of the nitrogen in...Ch. 23.5 - Prob. CQCh. 23.5 - The pKas of the conjugate acids of aniline and...Ch. 23.5 - Prob. EQ
Ch. 23.5 - Prob. FQCh. 23.5 - Prob. GQCh. 23.5 - Select the stronger acid from each pair of...Ch. 23.6 - Prob. 23.7PCh. 23.6 - Prob. 23.8PCh. 23.6 - Prob. 23.9PCh. 23.7 - Prob. 23.10PCh. 23.8 - Prob. 23.11PCh. 23.8 - Prob. 23.12PCh. 23.8 - Prob. 23.13PCh. 23.9 - Prob. 23.14PCh. 23.10 - In Example 23.15, you considered the product of...Ch. 23 - Prob. 23.16PCh. 23 - Prob. 23.17PCh. 23 - Prob. 23.18PCh. 23 - Prob. 23.19PCh. 23 - Prob. 23.20PCh. 23 - Prob. 23.21PCh. 23 - Prob. 23.22PCh. 23 - Account for the formation of the base peaks in...Ch. 23 - Prob. 23.24PCh. 23 - Select the stronger base from each pair of...Ch. 23 - The pKa, of the conjugate acid of morpholine is...Ch. 23 - Which of the two nitrogens in pyridoxamine (a form...Ch. 23 - Prob. 23.28PCh. 23 - Prob. 23.29PCh. 23 - Prob. 23.30PCh. 23 - Prob. 23.31PCh. 23 - Suppose you have a mixture of these three...Ch. 23 - Prob. 23.33PCh. 23 - Prob. 23.34PCh. 23 - Prob. 23.35PCh. 23 - Prob. 23.36PCh. 23 - Prob. 23.37PCh. 23 - (S)-Glutamic acid is one of the 20 amino acid...Ch. 23 - Prob. 23.39PCh. 23 - Propose a structural formula for the compound...Ch. 23 - Prob. 23.41PCh. 23 - The pyrolysis of acetic esters to give an alkene...Ch. 23 - Propose steps for the following conversions using...Ch. 23 - Show how to bring about each step in this...Ch. 23 - Show how to bring about each step in the following...Ch. 23 - Prob. 23.48PCh. 23 - Prob. 23.49PCh. 23 - Methylparaben is used as a preservative in foods,...Ch. 23 - Prob. 23.51PCh. 23 - Prob. 23.52PCh. 23 - Propose a synthesis for the systemic agricultural...Ch. 23 - Prob. 23.54PCh. 23 - Several diamines are building blocks for the...Ch. 23 - Prob. 23.56PCh. 23 - Prob. 23.57PCh. 23 - Prob. 23.58PCh. 23 - Prob. 23.59PCh. 23 - Following is a retrosynthesis for the coronary...Ch. 23 - Prob. 23.61PCh. 23 - Prob. 23.62PCh. 23 - Given this retrosynthetic analysis, propose a...Ch. 23 - Prob. 23.64PCh. 23 - Following is a series of anorexics (appetite...Ch. 23 - Prob. 23.66PCh. 23 - Prob. 23.67PCh. 23 - Show how the synthetic scheme developed in Problem...Ch. 23 - Prob. 23.69PCh. 23 - Prob. 23.70PCh. 23 - Prob. 23.71PCh. 23 - Prob. 23.72P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Indicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.arrow_forwardSynthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forward
- Synthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forward
- Indicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY