
Organic Chemistry-Package(Custom)
4th Edition
ISBN: 9781259141089
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 23, Problem 23.42P
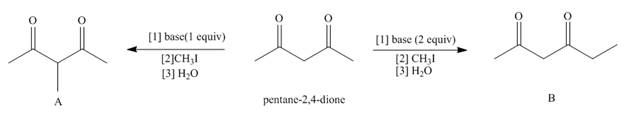
Explain why
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
please help by drawing the mechanism with arrows of this reaction please
Name the following molecules using iupac
Write the amididation reaction mechanism of a-aminophenol and acetic acid to produce acetaminophen
Chapter 23 Solutions
Organic Chemistry-Package(Custom)
Ch. 23 - Problem 23.1 Draw the enol or keto tautomer(s) of...Ch. 23 - Draw a stepwise mechanism for the following...Ch. 23 - Prob. 23.3PCh. 23 - Prob. 23.4PCh. 23 - Which CH bonds in the following molecules are...Ch. 23 - Prob. 23.6PCh. 23 - Prob. 23.7PCh. 23 - When ethyl acetoacetate (CH3COCH2CO2CH2CH3) is...Ch. 23 - Prob. 23.9PCh. 23 - Prob. 23.10P
Ch. 23 - Prob. 23.11PCh. 23 - Problem 23.11 Draw the products of each...Ch. 23 - Problem 23.12 Draw the products of each reaction....Ch. 23 - Prob. 23.14PCh. 23 - Prob. 23.15PCh. 23 - Prob. 23.16PCh. 23 - Prob. 23.17PCh. 23 - Prob. 23.18PCh. 23 - Problem 23.18 How can pentan-2-one be converted...Ch. 23 - Problem 23.19 Identify A, B, and C, intermediates...Ch. 23 - Prob. 23.21PCh. 23 - Problem 23.21 Draw the products of each...Ch. 23 - Prob. 23.23PCh. 23 - What alkyl halides are needed to prepare each...Ch. 23 - Prob. 23.25PCh. 23 - Prob. 23.26PCh. 23 - Prob. 23.27PCh. 23 - Prob. 23.28PCh. 23 - Prob. 23.29PCh. 23 - 23.29 Draw enol tautomer(s) for each compound....Ch. 23 - 22.30 The cis ketone A is isomerized to a trans...Ch. 23 - Draw enol tautomers for each compound. a. b. c.Ch. 23 - Prob. 23.33PCh. 23 - What hydrogen atoms in each compound have a pKa25?...Ch. 23 - Rank the labeled protons in each compound in order...Ch. 23 - Prob. 23.36PCh. 23 - Prob. 23.37PCh. 23 - Prob. 23.38PCh. 23 - Prob. 23.39PCh. 23 - 23.38 Acyclovir is an effective antiviral agent...Ch. 23 - Prob. 23.41PCh. 23 - 23.39 Explain why forms two different alkylation...Ch. 23 - 23.41 Acid-catalyzed bromination of pentanone ...Ch. 23 - Draw a stepwise mechanism for each reaction. a. b.Ch. 23 - What alkyl halides are needed to prepare each...Ch. 23 - Use the malonic ester synthesis to prepare each...Ch. 23 - 23.45 Devise a synthesis of valproic acid , a...Ch. 23 - Synthesize each compound from diethyl malonate....Ch. 23 - Prob. 23.49PCh. 23 - What alkyl halides are needed to prepare each...Ch. 23 - Synthesize each compound from ethyl acetoacetate....Ch. 23 - Draw the organic products formed in each reaction....Ch. 23 - 23.51 Draw the products formed (including...Ch. 23 - Prob. 23.54PCh. 23 - 23.54 Clopidogrel is the generic name for Plavix,...Ch. 23 - 23.55 What reaction conditions—base, solvent, and...Ch. 23 - Explain why each of the following reactions will...Ch. 23 - Prob. 23.58PCh. 23 - 23.57 Draw a stepwise mechanism showing how two...Ch. 23 - 23.58 Draw a stepwise mechanism for the following...Ch. 23 - Prob. 23.61PCh. 23 - 23.60 Draw stepwise mechanisms illustrating how...Ch. 23 - Prob. 23.63PCh. 23 - Prob. 23.64PCh. 23 - Convert acetophenone (C6H5COCH3) into each of the...Ch. 23 - Prob. 23.66PCh. 23 - Prob. 23.67PCh. 23 - Prob. 23.68PCh. 23 - Synthesize each product from ethyl acetoacetate...Ch. 23 - Prob. 23.70PCh. 23 - Prob. 23.71PCh. 23 - 23.68 Capsaicin, the spicy component of hot...Ch. 23 - 23.69 Treatment of W with , followed by , affords...Ch. 23 - Prob. 23.74PCh. 23 - Prob. 23.75PCh. 23 - Prob. 23.76PCh. 23 - Prob. 23.77PCh. 23 - Prob. 23.78P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forwardHow to draw the reaction mechasnism belowarrow_forwardName the following molecules with IUpacarrow_forward
- What is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?arrow_forwardUsing the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forwardHow to get the predicted product of this reaction belowarrow_forward
- Please help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forwardWrite the Esterification reaction mechanism for acetic acid, and one propanol to make propanol ethanoate (molecule that gives peas its odor in flavor)arrow_forwardProvide solutionsarrow_forward
- Which of these compounds is Ester formed from the reaction of acetic acid and one propanol arrow_forwardDescribe the four labeled parts of the reaction diagram from the reaction of sucrose, breaking down with and without an enzyme.arrow_forwardHow to determine the product with mechanism showedarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY