
Concept explainers
(a)
Interpretation:
The total number of molecules of NADH that are obtained by one run through the citric acid cycle is to be stated.
Concept introduction:
The cycle which represents the set of
Answer to Problem 23.28E
The total number of molecules of NADH that are obtained by one run through the citric acid cycle is three.
Explanation of Solution
The overall net equation that takes place in the citric acid cycle is given below.
In the above equation, the requirement of oxidizing agent such as FAD and
In third step, the oxidation of isocitrate into

Figure 1
In this step, the reduction of
In fourth step, the oxidation of the mixture of CoA-S-H and

Figure 2
In this step, the reduction of
In eighth step, the oxidation of malate into oxaloacetate takes place with the help of
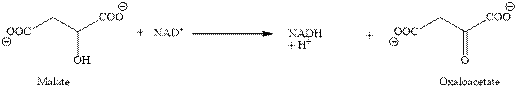
Figure 3
In this step also, the reduction of
Therefore, the third, fourth and eighth steps of citric acid cycle give total three molecules of NADH.
The total number of molecules of NADH that are obtained by one run through the citric acid cycle has been stated above.
(b)
Interpretation:
The total number of molecules of
Concept introduction:
The cycle which represents the set of chemical reactions that are utilized by all the aerobic or living organisms which helps them in releasing the stored amount of energy by the oxidation process of acetyl-CoA is known as citric acid cycle. This acetyl-CoA is obtained from the carbohydrates, protein and fats in the form of ATP.
Answer to Problem 23.28E
The total number of molecules of
Explanation of Solution
The sixth step of citric acid cycle includes the oxidation by FAD. In this step, the oxidation of succinate into fumarate takes place with the help of FAD as shown below.
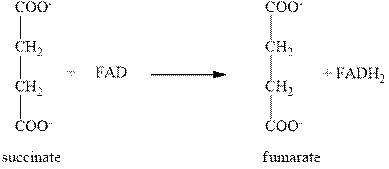
Figure 4
In this step, the reduction of FAD (flavin adenine dinucleotide) takes place to form
The total number of molecules of
(c)
Interpretation:
The total number of molecules of GTP that are obtained by one run through the citric acid cycle is to be stated.
Concept introduction:
The cycle which represents the set of chemical reactions that are utilized by all the aerobic or living organisms which helps them in releasing the stored amount of energy by the oxidation process of acetyl-CoA is known as citric acid cycle. This acetyl-CoA is obtained from the carbohydrates, protein and fats in the form of ATP.
Answer to Problem 23.28E
The total number of molecules GTP that are obtained by one run through the citric acid cycle is one.
Explanation of Solution
The fifth step of citric acid cycle includes the oxidation by GDP. In this step, the compound, succinyl CoA is converted into succinate with the help of GDP as shown below.
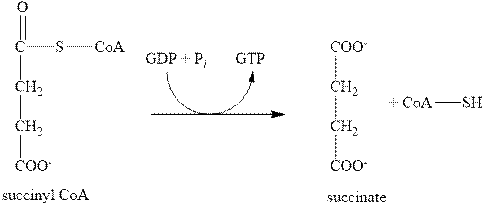
Figure 5
In this step, the GDP is also converted to one GTP molecule. Therefore, the fifth step of citric acid cycle gives only one molecule of GTP.
The total number of molecules of GTP that are obtained by one run through the citric acid cycle has been stated above.
(d)
Interpretation:
The total number of molecules of
Concept introduction:
The cycle which represents the set of chemical reactions that are utilized by all the aerobic or living organisms which helps them in releasing the stored amount of energy by the oxidation process of acetyl-CoA is known as citric acid cycle. This acetyl-CoA is obtained from the carbohydrates, protein and fats in the form of ATP.
Answer to Problem 23.28E
The total number of molecules
Explanation of Solution
The overall net equation that takes place in the citric acid cycle is given below.
According to the overall equation of citric acid cycle, total two molecules of
Therefore, the third and fourth steps of citric acid cycle give two molecules of
The total number of molecules of
Want to see more full solutions like this?
Chapter 23 Solutions
Bundle: Chemistry For Today: General, Organic, And Biochemistry, 9th + Owlv2 With Mindtap Reader, 1 Term (6 Months) Printed Access Card
- K Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward7 Comment on the general features of the predicted (extremely simplified) ¹H- NMR spectrum of lycopene that is provided below. 00 6 57 PPM 3 2 1 0arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning





