
Concept explainers
(a)
To determine: The mechanism that will increase or decrease insulin secretion by pancreatic
Introduction:
Insulin is the peptide hormone that is secreted by pancreas; insulin is produced naturally in the body of an organism. Insulin is required by the cells to use glucose as energy source. Glyburide is an antidiabetic drug that has sulfonylureas that close relation with sulfonamide antibiotics.
(a)
Explanation of Solution
Pictorial representation:
Fig 1: represent the pathway of glyburide:
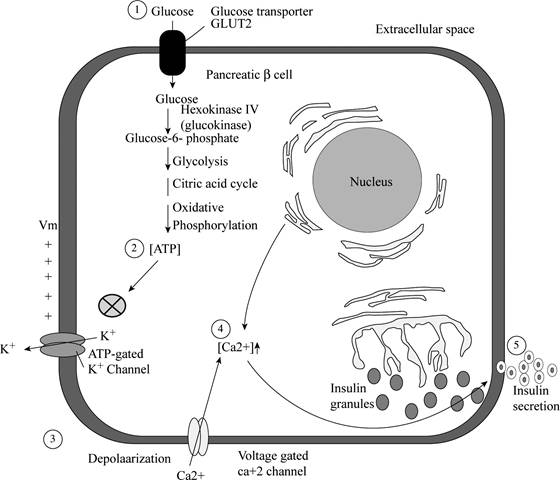
Fig.1: the pathway of glyburide
Explanation:
Treatment of diabetes with glyburide leads to the rapid secretion of insulin from the
Conclusion:
Glyburide will increase the secretion of insulin by shutting down the ATP-gated
(b)
To determine: The treatment process with glyburide that will reduce the symptoms of type 2 diabetes.
Introduction:
Glyburide is an antidiabetic drug that has sulfonylureas that has close relation with sulfonamide antibiotics. Type 2 diabetes is a severe case of diabetes that can be reversed by changing diet and lifestyle. To treat type 2 diabetes insulin is taken as injections..
(b)
Explanation of Solution
Explanation:
Glyburide drug will reduce the symptoms of type 2 diabetes because diabetes is a disease, which is cause due to the improper level of insulin. Glyburide will increase the secretion of insulin, and increased insulin level will reduces the symptoms of diabetes.
Conclusion:
Glyburide will reduce the symptoms of type 2 diabetes by increasing the insulin level in the body.
(c)
To determine: The reason that glyburide can be used to treat type 1 diabetes with explanation.
Introduction:
Glyburide is an antidiabetic drug that has sulfonylureas that close relation with sulfonamide antibiotic. Type 1 diabetes is an autoimmune condition in which immune system immune system remain active.
(c)
Explanation of Solution
Explanation:
Type 1, diabetes is caused by the defect in pancreatic cells. The glyburide drug is not useful for the Type 1 diabetes patients, because glyburide act on pancreatic cell to produce insulin, which maintain the level of glucose in the body.
Conclusion:
Type 1 diabetes occurs due to the defect in pancreatic cell. Glyburide drug is not useful for its treatment.
(d)
To determine: The importance of iodinated glyburide that has same binding characteristic with unaltered glyburide.
Introduction:
Insulin is the peptide hormone that is secreted by pancreas; insulin is produced naturally in the body of organism. Insulin is required by the cells to use glucose as energy source. Glyburide is an antidiabetic drug that has sulfonylureas that close relation with sulfonamide antibiotics.
(d)
Explanation of Solution
Pictorial representation:
Fig1: Represent the Iodinated glyburide:
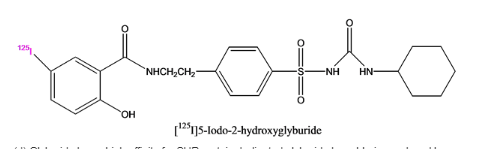
Explanation:
Glyburide show high affinity for SUR protein. Chlorine of glyburide is replaced by iodinated glyburide. Iodinated glyburide will not bind to SUR as glyburide does. If binding will not occur then wrong protein is cloned. This is required to differentiate iodinated glyburide with glyburide.
Conclusion:
Iodinated glyburide is required to differentiate iodinated glyburide with glyburide.
(e)
To determine: The reason for the requirement of antibody binding step.
Introduction:
Antibodies are also known as immunoglobulins, which are made up of proteins. These proteins will protect the living organism from the foreign substances. Glyburide is an antidiabetic drug that has sulfonylureas that close relation with sulfonamide antibiotics
(e)
Explanation of Solution
Explanation:
To purify the desired protein from a mixture of different proteins antibody-binding step is required. Antibody-binding step efficiently is used to separate desired protein from the mixture of different protein.
Conclusion:
Antibody binding step will separate out the desired protein from the mixture of different proteins.
(f)
To determine: The importance of putative SUR
Introduction:
Hybridization is the method that is used for the interbreeding of an individual from different population to produce hybrid. SUR subunit has 17 subunits that have 17 transmembrane domain.
(f)
Explanation of Solution
Explanation:
It is important to include putative SUR
Conclusion:
Hybridization step is important because cloned genes may code same sequences in another protein.
(g)
To determine: The name of
Introduction:
Gene cloning is the method by which similar copies of particular genes are produced. DNA cloning is a molecular technique that is used to make identical copies of the target gene.
(g)
Explanation of Solution
Explanation:
The following table represents the
| Experiment | Cell type | Added Putative SUR
|
Added excess unlabeled glyburide |
|
| 1 | HIT | No | No | +++ |
| 2 | HIT | No | Yes | - |
| 3 | No | No | - | |
| 4 | Yes | No | +++ | |
| 5 | Yes | Yes | - |
From the given table, the
Conclusion:
In the second experiment
(h)
To determine: The information from the given table to argue that
Introduction:
Gene cloning is the method by which similar copies of particular genes are produced. DNA cloning is a molecular technique that is used to make identical copies of the target gene.
(h)
Explanation of Solution
Explanation:
The
Conclusion:
The weight of cloned gene encodes SUR protein and weight as the SUR that is obtained from
(i)
To enlist: The extra information that is required to prove that SUR gene is cloned.
Introduction:
Gene cloning is the method by which similar copies of particular genes are produced. DNA cloning is a molecular technique that is used to make identical copies of the target gene.
(i)
Explanation of Solution
Explanation:
To prove that SUR gene is cloned following information are required:
1) Check whether the transformed cells have ATP- gated
2) ATP- gated
3) Check whether these organisms are able to produce insulin by introducing mutants of the putative SUR gene.
Conclusion:
To prove that SUR gene is cloned, ATP-gated
Want to see more full solutions like this?
Chapter 23 Solutions
EBK LEHNINGER PRINCIPLES OF BIOCHEMISTR
- what is a protein that contains a b-sheet and how does the secondary structure contributes to the overall function of the protein.arrow_forwarddraw and annotate a b-sheet and lable the hydrogen bonding. what is an example that contains the b-sheet and how the secondary structure contributes to the overall function of your example protein.arrow_forwardFour distinct classes of interactions (inter and intramolecular forces) contribute to a protein's tertiary and quaternary structures. Name the interaction then describe the amino acids that can form this type of interaction. Draw and annotate a diagram of the interaction between two amino acids.arrow_forward
- Examine the metabolic pathway. The enzymes that catalyze each step are identified as "e" with a numeric subscript. e₁ e3 e4 A B с 1° B' 02 e5 e6 e7 E F Which enzymes catalyze irreversible reactions? ப e ez ☐ ez e4 ☐ ப es 26 5 e7 Which of the enzymes is likely to be the allosteric enzyme that controls the synthesis of G? €2 ез e4 es 26 5 e7arrow_forwardAn allosteric enzyme that follows the concerted model has an allosteric coefficient (T/R) of 300 in the absence of substrate. Suppose that a mutation reversed the ratio. Select the effects this mutation will have on the relationship between the rate of the reaction (V) and substrate concentration, [S]. ㅁㅁㅁ The enzyme would likely follow Michaelis-Menten kinetics. The plot of V versus [S] would be sigmoidal. The enzyme would mostly be in the T form. The plot of V versus [S] would be hyperbolic. The enzyme would be more active.arrow_forwardPenicillin is hydrolyzed and thereby rendered inactive by penicillinase (also known as ẞ-lactamase), an enzyme present in some penicillin-resistant bacteria. The mass of this enzyme in Staphylococcus aureus is 29.6 kDa. The amount of penicillin hydrolyzed in 1 minute in a 10.0 mL. solution containing 1.00 x 10 g of purified penicillinase was measured as a function of the concentration of penicillin. Assume that the concentration of penicillin does not change appreciably during the assay. Plots of V versus [S] and 1/V versus 1/[S] for these data are shown. Vo (* 10 M minute"¹) 7.0 6.0 5.0 4.0 3.0 20 1.0 0.0 о 10 20 30 1/Vo (* 10 M1 minute) 20 103 90 BO 70 50 [S] (* 100 M) 40 50 60 y=762x+1.46 × 10" [Penicillin] (M) Amount hydrolyzed (uM) 1 0.11 3 0.25 5 0.34 10 0.45 30 0.58 50 0.61arrow_forward
- Consider the four graphs shown. In each graph, the solid blue curve represents the unmodified allosteric enzyme and the dashed green curve represents the enzyme in the presence of the effector. Identify which graphs correctly illustrate the effect of a negative modifier (allosteric inhibitor) and a positive modifier (allosteric activator) on the velocity curve of an allosteric enzyme. Place the correct graph in the set of axes for each type of modifier. Negative modifier Reaction velocity - Positive modifier Substrate concentration - Reaction velocity →→→→ Substrate concentration Answer Bankarrow_forwardConsider the reaction: phosphoglucoisomerase Glucose 6-phosphate: glucose 1-phosphate After reactant and product were mixed and allowed to reach at 25 °C, the concentration of each compound at equilibrium was measured: [Glucose 1-phosphate] = 0.01 M [Glucose 6-phosphate] = 0.19 M Calculate Keq and AG°'. Код .0526 Incorrect Answer 7.30 AG°' kJ mol-1 Incorrect Answerarrow_forwardClassify each phrase as describing kinases, phosphatases, neither, or both. Kinases Phosphatases Neither Both Answer Bank transfer phosphoryl groups to acidic amino acids in eukaryotes may use ATP as a phosphoryl group donor remove phosphoryl groups from proteins catalyze reactions that are the reverse of dephosphorylation reactions regulate the activity of other proteins catalyze phosphorylation reactions PKA as an example turn off signaling pathways triggered by kinasesarrow_forward
- Consider the reaction. kp S P kg What effects are produced by an enzyme on the general reaction? AG for the reaction increases. The rate constant for the reverse reaction (kr) increases. The reaction equilibrium is shifted toward the products. The concentration of the reactants is increased. The activation energy for the reaction is lowered. The formation of the transition state is promoted.arrow_forwardThe graph displays the activities of wild-type and several mutated forms of subtilisin on a logarithmic scale. The mutations are identified as: • The first letter is the one-letter abbreviation for the amino acid being altered. • The number identifies the position of the residue in the primary structure. ⚫ The second letter is the one-letter abbreviation for the amino acid replacing the original one. • Uncat. refers to the estimated rate for the uncatalyzed reaction. Log₁(S-1) Wild type S221A H64A -5 D32A S221A H64A D32A -10 Uncat. How would the activity of a reaction catalyzed by a version of subtilisin with all three residues in the catalytic triad mutated compare to the activity of the uncatalyzed reaction? It would have more activity, because the reaction catalyzed by the triple mutant is approximately three-fold faster than the uncatalyzed reaction. It would have less activity, because the reaction catalyzed by the triple mutant is approximately 1000-fold slower than the…arrow_forwardB Substrate Product AL Product Substrate Reaction progress- Reaction progress- omplete the passage describing the two reactions. In reaction A, the stability of the substrate is (AG) of the reaction is positive, Incorrect Answer greater than the stability of the product. The free-energy change Incorrect Answer so the reaction is considered In reaction B, the stability of the substrate is (AG) of the reaction is less than Incorrect Answer endergonic and Incorrect Answer not spontaneous. Incorrect Answer the stability of the product. The free-energy change negative, so the reaction is considered Incorrect Answer exergonic and spontaneous. Incorrect Answer Incorrect Answerarrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





