
(a)
To determine: The experiment to confirm the formation of
(a)
Answer to Problem 1DE
Solution: The reaction of the given product with
Explanation of Solution
The reaction of the given coordination compound with the solution of
Whereas, if the compound formed is
This will confirm the formation of desired product.
The reaction of the given product with
(b)
To determine: If the cobalt present in the given reaction is in
(b)
Answer to Problem 1DE
Solution: The oxidation state of cobalt is
Explanation of Solution
The oxidation state of cobalt in
Substitute the values of oxidation state of ethylene diammine, chlorine and the total charge on the molecule in the above equation,
Therefore, the oxidation state of cobalt in
The electronic configuration of
The ethylene diammine present in the given complex is a strong field ligand which causes pairing of electrons in metal ions.
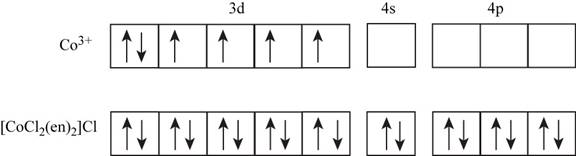
Figure 1
Since, there is no unpaired electron present in the given complex. Therefore, the spin state of the cobalt is zero in the given complex.
The oxidation state of cobalt is
(c)
To determine: The existence of geometrical isomerism in the complex
(c)
Answer to Problem 1DE
Solution: The geometrical isomerism exists in the given complex and the isomers are of different colors which confirm their presence in the mixture.
Explanation of Solution
The geometrical isomerism is the isomerism in which the structure and molecular formula of the molecule is same but the orientation of groups around the metal atoms changes.
The molecule
The geometrical isomers of the
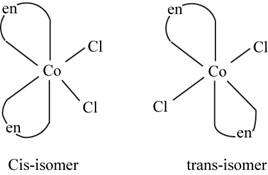
Figure 2
The isomers of
The geometrical isomerism exists in the given complex and the isomers are of different colors which confirm their presence in the mixture.
(d)
To determine: The way to know the isomer which is formed as a product in the given reaction out of the two isomers.
(d)
Answer to Problem 1DE
Solution: The color of trans isomer is green and of cis isomer is violet which gives the confirmation of the geometry of product.
Explanation of Solution
The color of cis isomer of
The color of trans isomer is green and of cis isomer is violet which gives the confirmation of the geometry of product.
Want to see more full solutions like this?
Chapter 23 Solutions
Chemistry: The Central Science (14th Edition)
- Write the systematic name of each organic molecule: structure HO-C-CH2-CH3 O -OH CH3-CH2-CH2-CH2-CH2-C-OH CH3 CH3-CH-CH2-C-OH Explanation Check S namearrow_forwardtheres 2 productsarrow_forwardDraw the major product of this solvolysis reaction. Ignore any inorganic byproducts. + CH3CH2OH Drawing Q Atoms, Bonds and Rings OCH2CH3 || OEt Charges OH 00-> | Undo Reset | Br Remove Done Drag To Pan +arrow_forward
- Draw the major product of this SN1 reaction. Ignore any inorganic byproducts. CH3CO2Na CH3CO2H Drawing + Br Q Atoms, Bonds and Rings OAC Charges OH ОАс Na ဂ Br Undo Reset Remove Done Drag To Pan +arrow_forwardOrganic Functional Groups entifying positions labeled with Greek letters in acids and derivatives 1/5 ssible, replace an H atom on the a carbon of the molecule in the drawing area with a ce an H atom on the ẞ carbon with a hydroxyl group substituent. ne of the substituents can't be added for any reason, just don't add it. If neither substi er the drawing area. O H OH Oneither substituent can be added. Check D 1 Accessibility ado na witharrow_forwardDifferentiate between electrophilic and nucleophilic groups. Give examples.arrow_forward
- An aldehyde/ketone plus an alcohol gives a hemiacetal, and an excess of alcohol gives an acetal. The reaction is an equilibrium; in aldehydes, it's shifted to the right and in ketones, to the left. Explain.arrow_forwardDraw a Haworth projection or a common cyclic form of this monosaccharide: H- -OH H- OH H- -OH CH₂OHarrow_forwardAnswer the question in the first photoarrow_forward
- Ggggffg2258555426855 please don't use AI Calculate the positions at which the probability of a particle in a one-dimensional box is maximum if the particle is in the fifth energy level and in the eighth energy level.arrow_forwardExplain the concepts of hemiacetal and acetal.arrow_forwardBriefly describe a nucleophilic addition.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





