
The IUPAC rules permit the use of common names for a number of familiar phenols and aryl ethers. These common names are listed here along with their systematic names. Write the structure of each compound.
(a)
(b)
(c)
(d)
(e)
(f)
Interpretation:
The structures of the given compounds are to be drawn.
Concept introduction:
The systematic naming of organic compound is done by using IUPAC nomenclature. The naming of an organic compound is done in such a way that the structure of the organic compound is correctly interpreted from its name.
In order to determine the structure of an organic compound from its name, first the root word in the name is identified. The suffixes like
In the next step of structure identification of the organic compound from its name, the position, location and number of the substituents bonded to the carbon chain are determined.
Answer to Problem 12P
Solution:
a) The structure of
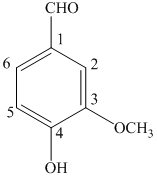
b) The structure of
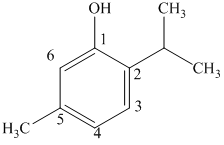
c) The structure of
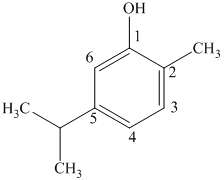
d) The structure of
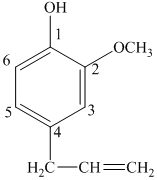
e) The structure of
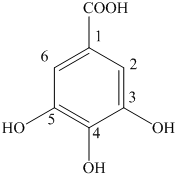
f) The structure of
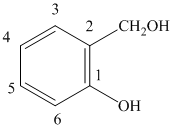
Explanation of Solution
a) The structure of
The given compound is

b) The structure of
The given compound is

c) The structure of
The given compound is

d) The structure of
The given compound is

e) The structure of
The given compound is

f) The structure of is
The given compound is

Want to see more full solutions like this?
Chapter 23 Solutions
Organic Chemistry - Standalone book
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
- er your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward5.arrow_forward6.arrow_forward
